PEDIATRIC SURGERY UPDATE ©
VOLUME 41, 2013
PSU Volume 41 No 01 JULY 2013
Acquired Jeune's Syndrome
Jeune's syndrome, also known as
asphyxiating thoracic dysplasia, is a type of thoracic dystrophy with
severe narrow thorax leading to respiratory distress and even death in
its more severe form. Jeune's syndrome either is congenital (autosomal
recessive) or acquired. The acquired form of Jeune's syndrome was
described in 1996 in children who had undergone repair of pectus
excavatum chest wall deformity utilizing the traditional open (Ravitch)
approach with subperichondrial resection of deformed cartilages and
transverse osteotomy performed too early an age (less than four years
of age). Permanent impairment of normal chest wall growth and
subsequent restriction of lung expansion during respiration creates
this type of acquired form of the disease. Years later after the
primary procedure for pectus these children developed progressive
dyspnea with mild exertion associated to restrictive pulmonary function
tests with forced vital capacity (FVC) of 30-50% and forced expiratory
volume in one second (FEV1) of 30-60% of predicted values. This
acquired restrictive thoracic dystrophy is due to an aggressive
resection of the involved deformed cartilages including the second
costal cartilage. This complication does not occur using the actual
minimally invasive repair of pectus excavatum (Nuss technique).
Diagnosis is done using pulmonary function tests and three-dimensional
CT reconstruction of the chest. Management of acquired Jeune's syndrome
includes displacing the sternum forward with a splint or median
sternotomy with interposition of autologous rib grafts to increase the
chest wall diameter (Weber technique). Substantial improvement in PFT
and clinical symptoms can be achieved with the sternal split technique
though long-term evaluation is awaiting results.
References:
1- de Vries J, Yntema JL, van Die CE, Crama N, Cornelissen EA, Hamel
BC: Jeune syndrome: description of 13 cases and a proposal for
follow-up protocol. Eur J Pediatr. 169(1):77-88, 20102- Weber TR,
Kurkchubasche AG: Operative management of asphyxiating thoracic
dystrophy after pectus repair. J Pediatr Surg. 33(2):262-5, 19983-
Fokin AA, Robicsek F: Acquired deformities of the anterior chest wall.
Thorac Cardiovasc Surg. 54(1):57-61, 20064- Weber TR: Further
experience with the operative management of asphyxiating thoracic
dystrophy after pectus repair. J Pediatr Surg. 40(1):170-3, 20055-
Phillips JD, van Aalst JA: Jeune's syndrome (asphyxiating thoracic
dystrophy): congenital and acquired. Semin Pediatr Surg. 17(3):167-72,
2008 6- Lopushinsky SR, Fecteau AH: Pectus deformities: a review of
open surgery in the modern era. Semin Pediatr Surg. 17(3):201-8, 2008
Metal Allergy
Jewelry, dental and surgical implants
from craniofacial, orthopedic, neurosurgical and pediatric surgery
physicians can lead to metal allergy in children. As many as 13% of
patients are sensitive to nickel, cobalt or chromium. Metal allergy
from nickel is the most common contact allergy in the United States and
Europe. The classical symptom of dermatitis caused by nickel is a rash
in the earlobes, periumbilical region or wrist resulting from contact
with costume jewelry, buttons and zipper. Metal allergy is a typical
delayed type IV hypersensitivity reaction caused by T-lymphocytes
reaction. CD8 and CD4 cells cause cytotoxic and inflammatory response
to the metal. Children with metal allergy usually elicit a past history
of atopy including allergic rhinitis, asthma, eczema and urticarial
rash. Metal allergies are frequently misdiagnosed as surgical
infections. Symptoms of inflammation such as pain, warmth, erythema and
swelling can be seen over the implant, including pericarditis and
pleural effusion in those in a thoracic position. As a screening
measure to determine if a child can or might develop metal allergy to
an implant the following should be evaluated: 1- history of allergy to
jewelry, orthodontic braces, metal buttons on clothing and food. 2-
History of previous atopy and eczema. If any of the above indications
are found, a dermal patch test should be performed. This patch test
contains 23 allergens and allergen mixes that cause up to 80% of
allergic contact dermatitis cases. Should the child be found to have
metal allergy implants of titanium should be considered, since titanium
does not produce allergic reactions but are more
expensive.
References:
1- Mortz CG, Lauritsen JM, Bindslev-Jensen C, Andersen KE:
Nickel sensitization in adolescents and association with ear piercing,
use of dental braces and hand eczema. The Odense Adolescence Cohort
Study on Atopic Diseases and Dermatitis (TOACS). Acta Derm Venereol.
82(5):359-64, 2002
2- Dotterud LK, Falk ES: Metal allergy in north Norwegian
schoolchildren and its relationship with ear piercing and atopy.
Contact Dermatitis. 31(5):308-13, 1994
3- Kalimo K, Mattila L, Kautiainen H: Nickel allergy and orthodontic treatment. J Eur Acad Dermatol Venereol. 18(5):543-5, 2004
4- Katting B, Brehler R, Traupe H: Allergic contact dermatitis
in children: strategies of prevention and risk management. Eur J
Dermatol. 14(2):80-5, 2004
5- Rushing GD, Goretsky MJ, Gustin T, Morales M, Kelly RE Jr,
Nuss D: When it is not an infection: metal allergy after the Nuss
procedure for repair of pectus excavatum. J Pediatr Surg. 42(1):93-7,
2007
6- Thyssen JP, Jakobsen SS, Engkilde K, Johansen JD,
SA¸balle K, Menna T: The association between metal allergy, total
hip arthroplasty, and revision. Acta Orthop. 80(6):646-52, 2009
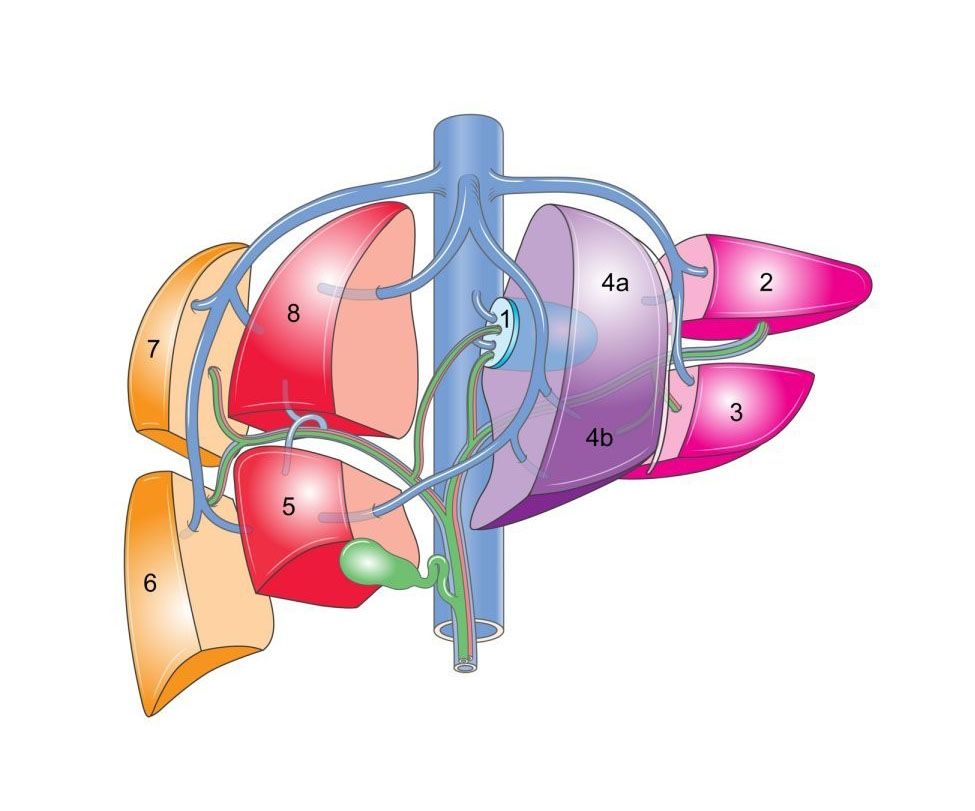
PRETEXT
The PRETEXT (PRE Treatment EXTent of
disease) system was designed by the International Childhood Liver Tumor
Strategy Group (SIOPEL) for staging and risk stratification of liver
tumors, namely hepatoblastoma, hepatocellular carcinoma and epithelioid
hemangioendothelioma. PRETEXT describes tumor extent before any therapy
allowing different groups to have a more effective comparison in future
studies. PRETEXT staging is based on Couinaud's liver segmentation
grouping the liver into four sections: segment 2 and 3 (left lateral
section), segment 4a and 4b (left medial section), segments 5 and 8
(right anterior section) and segments 6 and 7 (right posterior
section). The PRETEXT number is derived by subtracting the highest
number of contiguous liver sections that are not involved by tumor from
four. PRETEXT also utilizes other criteria such as involvement of the
caudate lobe (designated C), involvement of the inferior vena cava or
hepatic veins (V), involvement of the portal veins (P), extrahepatic
abdominal disease (E) and distant metastasis (M). Other high risk
criteria include tumor rupture or intraperitoneal hemorrhage at
diagnosis (H1) and alpha fetoprotein levels below 100 ug/L. In PRETEXT
I one section is involved and three are free. This group includes only
a small portion of all cases. In PRETEXT II one or two sections re
involved, but two adjoining sections are free. They are limited to the
right lobe or left lobe of the liver. In PRETEXT III two or three
sections are involved and no two adjoining sections are free. The
unifocal tumors in this category spare only the left lateral or right
posterior section. This group is relatively common. In PRETEXT IV all
four sections are involved. Involvement of the caudate lobe is a
potential predictor of a poor outcome. Extrahepatic disease refers to
diaphragm involvement, peritoneal seeding, ascites and abdominal lymph
node metastasis. Distant metastasis is manly to the
lung.
References:
1- Brown J, Perilongo G, Shafford E, Keeling J, Pritchard J,
Brock P, Dicks-Mireaux C, Phillips A, Vos A, Plaschkes J: Pretreatment
prognostic factors for children with hepatoblastoma-- results from
the International Society of Paediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer. 36(11):1418-25, 2000
2- Perilongo G, Shafford E, Plaschkes J; Liver Tumour Study
Group of the International Society of Paediatric Oncology: SIOPEL
trials using preoperative chemotherapy in hepatoblastoma. Lancet Oncol.
1:94-100, 2000
3- Roebuck DJ, Aronson D, Clapuyt P, Czauderna P, de Ville de
Goyet J, Gauthier F, Mackinlay G, Maibach R, McHugh K, Olsen OE, Otte
JB, Pariente D, Plaschkes J, Childs M, Perilongo G; International
Childrhood Liver Tumor Strategy Group: 2005 PRETEXT: a revised staging
system for primary malignant liver tumours of childhood developed by
the SIOPEL group. Pediatr Radiol. 37(2):123-32, 2007
4- Roebuck DJ: Assessment of malignant liver tumors in children. Cancer Imaging. 9: S98-S103, 2009
5- Meyers RL, Czauderna P, Otte JB: Surgical treatment of hepatoblastoma. Pediatr Blood Cancer. 59(5):800-8, 2012
6- Tajiri T, Kimura O, Fumino S, Furukawa T, Iehara T, Souzaki
R, Kinoshita Y, Koga Y, Suminoe A, Hara T, Kohashi K, Oda Y,
Hishiki T, Hosoi H, Hiyama E, Taguchi T: Surgical strategies for
unresectable hepatoblastomas. J Pediatr Surg. 47(12):2194-8, 2012
PSU Volume 41 No 02 AUGUST 2013
Duhamel Procedure
In 1956 Bernard Duhamel first
described a rectorectal pull-through procedure. Since then, the Duhamel
is a long standing pull-through procedure performed for the management
of Hirschsprung's disease (HD). In short, the procedure entails pulling
the proximal normal ganglionic bowel posterior to the aganglionic
rectum through the presacral space into the anus. A common lumen is
created with mechanical devices between the ganglionic and aganglionic
rectal bowel. The surgical approach to HD has changed from building an
initial temporary colostomy using a two or even three stage procedures,
to a one-stage surgical procedure in the neonatal period. As with any
other kind of surgical procedure for Hirschsprung's disease the child
can develop postoperative constipation, soiling or incontinence.
Constipation and soiling after the Duhamel procedure often are
associated with an anterior rectal pouch caused by a colorectal spur.
Constipation also may result from outlet obstruction caused by residual
spasticity of the internal sphincter or too long rectal aganglionic
bowel. Extended resection of the aganglionic rectum reduces the
incidence of fecalomas formation. Suboptimal outcome after operation
for Hirschsprung's' disease includes associated neuronal intestinal
dysplasia, total colonic involvement, significant neurological
impairment and history of enterocolitis all of which may result in
abnormal colonic motility in the remaining ganglionic bowel. Chronic
bleeding after Duhamel is caused by an incomplete section of the septum
between rectum and pull-through segment leaving the feeding artery on
the tip of the side-to-side anastomosis. After rectosigmoidectomy
alteration in bladder function such as increase bladder capacity and
urinary residual has been described. Open and laparoscopic Duhamel
procedure has similar outcomes.
References:
1- Baillie CT, Kenny SE, Rintala RJ, Booth JM, Lloyd DA: Long-term
outcome and colonic motility after the Duhamel procedure for
Hirschsprung's disease.J Pediatr Surg. 34(2):325-9, 1999
2- van der Zee DC, Bax KN: One-stage Duhamel-Martin procedure for
Hirschsprung's disease: a 5-year follow-up study. J Pediatr Surg.
35(10):1434-6, 2000
3- Mattioli G, Castagnetti M, Martucciello G, Jasonni V: Results of a
mechanical Duhamel pull-through for the treatment of
Hirschsprung's disease and intestinal neuronal dysplasia. J
Pediatr Surg. 39(9):1349-55, 2004
4- Chiengkriwate P, Patrapinyokul S, Sangkhathat S, Chowchuvech V:
Primary pull-through with modified Duhamel technique: 1 institution's
experience. J Pediatr Surg. 42(6):1075-80, 2007
5- Marquez TT, Acton RD, Hess DJ, Duval S, Saltzman DA: Comprehensive
review of procedures for total colonic aganglionosis. J Pediatr Surg.
44(1):257-65, 2009
6- Nah SA, de Coppi P, Kiely EM, Curry JI, Drake DP, Cross K, Spitz L,
Eaton S, Pierro A: Duhamel pull-through for Hirschsprung disease: a
comparison of open and laparoscopic techniques. J Pediatr Surg.
47(2):308-12, 2012
Prevention of NEC
Necrotizing enterocolitis (NEC) is the
most common surgical emergency in the neonatal intensive care unit
(NICU) associated with a significant morbidity and mortality. Due to
advances in neonatal care survival among premature and low birth weight
infants has improved though the mortality due to NEC has increased.
Prevention of NEC should be of upmost importance in most NICU. One of
the most important preventive measures in the development of NEC is
feeding infants at risk with breast milk as opposed to formula milk. If
fortification of breast milk is necessary to achieve adequate growth,
then a fortifier based on human milk lowers the incidence of NEC.
Another factor associated with lowering the incidence of NEC is whether
to use early or delayed enteral feedings. Current metaanalysis does not
support the use of a delayed introduction or slower rate of enteral
feeds to prevent NEC. They slower the weight gain and take longer time
to full feeding. Adverse effect of immunoglobulin administration or
prophylactic enteral antibiotics precluded their use as preventive
measure. Prophylactic antibiotics increase the colonization of
resistant bacteria. The other measure with strong evidence to use for
NEC prevention is the administration of probiotics and modulation of
feeding regimens in infants at high risk. Studies investigating
specific components of breast milk and probiotics responsible for these
protective effects have identified several molecules with therapeutic
potential such as erythropoietin, glutamine, and epidermal growth
factor all of which strengthen the gut barrier. Probiotics reduce
intestinal permeability, promote peristalsis, increase mucin secretion,
activate anti-inflammatory TLR9, downregulate proinflammatory cytokines
and upregulated antiinflammatory mediators.
References:
1- Morgan JA, Young L, McGuire W: Pathogenesis and prevention of
necrotizing enterocolitis. Curr Opin Infect Dis. 24(3):183-9, 2011
2- Berman L, Moss RL: Necrotizing enterocolitis: an update. Semin Fetal Neonatal Med. 16(3):145-50, 2011
3- Ganguli K, Meng D, Rautava S, Lu L, Walker WA, Nanthakumar N:
Probiotics prevent necrotizing enterocolitis by modulating enterocyte
genes that regulate innate immune-mediated inflammation. Am J
Physiol Gastrointest Liver Physiol. 304(2):G132-41, 2013
4- Downard CD, Renaud E, St Peter SD, Abdullah F, Islam S, Saito JM,
Blakely ML, Huang EY, Arca MJ, Cassidy L, Aspelund G; 2012 American
Pediatric Surgical Association Outcomes Clinical Trials Committee.
Treatment of necrotizing enterocolitis: an American Pediatric Surgical
Association Outcomes and Clinical Trials Committee systematic review. J
Pediatr Surg. 47(11):2111-22, 2012
5- Bernardo WM, Aires FT, Carneiro RM, Sá FP, Rullo VE,
Burns DA: Effectiveness of probiotics in the prophylaxis of necrotizing
enterocolitis in preterm neonates: a systematic review and
meta-analysis. J Pediatr (Rio J). 89(1):18-24, 2013
6- Raval MV, Hall NJ, Pierro A, Moss RL: Evidence-based prevention and
surgical treatment of necrotizing enterocolitis-a review of randomized
controlled trials. Semin Pediatr Surg. 22(2):117-21, 2013
Music-Induced Stress Reduction
Music intervention has been found to
reduce procedure related anxiety for patients in the pre- and
intraoperative setting. Studies have shown that music reduced
self-reported stress levels and improve perceptions of patient-oriented
service in visitors to the surgery/intensive care unit waiting room.
Adults who listened to music while waiting with their children in the
pediatric emergency department, reported lower anxiety level than those
who did not listen to music. The level of formal education is inversely
correlated to degree of music-induced anxiety reduction. Music improved
the work environment for hospital staff and facilitated their
interactions with friends and family of patients. Allowing patients
control over music selection and providing uninterrupted time for music
listening gives the patients an enhanced sense of control in an
environment that often controls them. Music alone and music assisted
relaxation techniques significantly decreased arousal due to stress.
Further analysis revealed that the amount of stress reduction was
significantly different when considering age, type of stress, music
assisted relaxation technique, musical preference, previous music
experience, and type of intervention. Music specifically induces an
emotional response similar to a pleasant experience or happiness. Music
in the operating room has immeasurable effects. It can prevent
distraction, minimize annoyance, reduce stress, reduce the demands for
analgesic and anesthetics, and diminish the anxiety of patients, staff
and users.
References:
1- Pelletier CL: The effect of music on decreasing arousal due to
stress: a meta-analysis. J Music Ther. 41(3):192-214, 2004
2- Suda M, Morimoto K, Obata A, Koizumi H, Maki A: Emotional responses
to music: towards scientific perspectives on music therapy.
Neuroreport. 19(1):75-8, 2008
3- Makama JG, Ameh EA, Eguma SA: Music in the operating theatre:
opinions of staff and patients of a Nigerian teaching hospital. Afr
Health Sci. 10(4):386-9, 2010
4- Moris DN, Linos D: Music meets surgery: two sides to the art of "healing". Surg Endosc. 27(3):719-23, 2013
5- Tilt AC, Werner PD, Brown DF, Alam HB, Warshaw AL, Parry BA, Jazbar
B, Booker A, Stangenberg L, Fricchione GL, Benson H, Lillemoe KD,
Conrad C: Low degree of formal education and musical experience predict
degree of music-induced stress reduction in relatives and friends of
patients: a single-center, randomized controlled trial. Ann Surg.
257(5):834-8, 2013
6- Beaulieu-Boire G, Bourque S, Chagnon F, Chouinard L, Gallo-Payet N,
Lesur O: Music and biological stress dampening in
mechanically-ventilated patients at the intensive care unit
ward-a prospective interventional randomized crossover trial. J Crit
Care. Mar 14, 2013
PSU Volume 41 No 03 SEPTEMBER 2013
Octreotide
Octreotide is a synthetic peptide
analog of somatostatin with the same pharmacologic effect. Octreotide
has a longer half-life in circulation and higher potency than
somatostatin. Octreotide decreases the production of gastrointestinal
peptides, such as gastrin, secretin, gastric inhibitory peptide,
cholecystokinin, neurotensin, motilin, and pancreatic polypeptide.
Octreotide has several therapeutic uses in children. Octreotide
significantly reduced the amount of blood transfusions in children with
severe gastrointestinal bleeding and hemodynamic instability from acute
variceal hemorrhage. Octreotide successfully reduced bleeding in a
patient with typhlitis and cecal ulceration prior to surgery but failed
to control massive bleeding in children with a Meckel's diverticulum.
Octreotide inhibits pancreatic secretion and can be of help in
resolution of pancreatic pseudocysts allowing healing of pancreatic
duct with resolution of ascites. It can significantly reduce serum
lipase levels and reduce the clinical need for analgesics in acute
pancreatitis. Octreotide is effective in the management of chylothorax
by shortening the TPN duration, hospital stay and avoiding surgery
since it reduces thoracic duct lymph flow and absorption. Octreotide is
effective in reducing stool output in children with a variety of
disorders, including massive ileostomy losses, intestinal fistula,
congenital microvillus atrophy, idiopathic secretory diarrhea,
carcinoid tumor, cryptosporidium diarrhea and watery diarrhea
hypokalemia achlorhydria syndrome. Octreotide should be avoided in
patients with diagnosed or suspected congenital long Q–Tc
syndrome and cautiously used in conjunction with drugs that prolong
Q–T interval. Because of its inhibitory action on insulin,
octreotide has been associated with glucose intolerance and
hyperglycemia that may necessitate insulin therapy.
References:
1- Paget-Brown A, Kattwinkel J, Rodgers BM, Michalsky MP: The use of
octreotide to treat congenital chylothorax. J Pediatr Surg.
41(4):845-7, 2006
2- Al-Hussaini A, Butzner D: Therapeutic applications of octreotide in
pediatric patients. Saudi J Gastroenterol. 18(2):87-94, 2012
3- Nardone G, Rocco A, Balzano T, Budillon G: The efficacy of
octreotide therapy in chronic bleeding due to vascular abnormalities of
the gastrointestinal tract. Aliment Pharmacol Ther. 13(11):1429-36, 1999
4- Heikenen JB, Pohl JF, Werlin SL, Bucuvalas JC: Octreotide in
pediatric patients. J Pediatr Gastroenterol Nutr. 35(5):600-9, 2002
5- Sahin Y, Aydin D: Congenital chylothorax treated with octreotide. Indian J Pediatr. 72(10):885-8, 2005
6- Helin RD, Angeles ST, Bhat R: Octreotide therapy for chylothorax in
infants and children: A brief review. Pediatr Crit Care Med.
7(6):576-9, 2006
Peritoneovenous Shunt
Peritoneovenous shunt (PVS), also
known as Leveen or Denver shunt, is a shunt utilized to manage
medically intractable ascites in adults and children. These shunts
allow ascitic fluid to flow down a pressure gradient from the
peritoneal cavity to the venous circulation and have a valve mechanism
that prevents backflow of blood if the venous pressure rises above the
intraabdominal pressure. The advantage of the Denver shunt is that the
valve chamber lies in the subcutaneous tissue and therefore can be
manually compressed to relieve blockage and promote flow. The shunt can
also be flushed percutaneously if necessary. When peritoneal pressure
is 3 cm higher than CV pressure the valve opens. PVS can be placed
surgically, laparoscopically-assisted or percutaneously. Persistent
ascites is rare in children, carries a significant morbidity and is a
difficult management problem owing to the massive abdominal distension.
Etiology is often related to previous surgery, congenital malformation
of lymphatic channels, or idiopathic. Other causes include
inflammatory, neoplastic, traumatic, mechanical obstruction or
nonaccidental injury. Conservative and symptomatic management is
usually the mainstay of treatment while surgery is indicated when
conservative therapy fails. Certain complications have been described
in association with the procedure of placing the PVS. One of the major
concerns is diversion of large amounts of fluid into the central
circulation, potentially contributing to fluid overload. In
anticipation of this potential complication, diuretic therapy is
initiated that seemed to avoid this problem. Other reported
complications include shunt blockage and leaks, venous thrombosis,
disseminated intravascular coagulation, infection, and air embolus
during insertion. The PVS is an effective alternative in management for
intractable ascites in children.
References:
1- Blaylock RS, Emby D, Hopley M, Toogood JW: The peritoneo-saphenous
shunt for palliation of refractory ascites. S Afr J Surg. 39(3):83-5,
2001
2- Sooriakumaran P, McAndrew HF, Kiely EM, Spitz L, Pierro A:
Peritoneovenous shunting is an effective treatment for intractable
ascites. Postgrad Med J. 81(954):259-61, 2005
3- Matsufuji H, Nishio T, Hosoya R: Successful treatment for
intractable chylous ascites in a child using a peritoneovenous shunt.
Pediatr Surg Int. 22(5):471-3, 2006
4- Won JY, Choi SY, Ko HK, Kim SH, Lee KH, Lee JT, Lee do Y:
Percutaneous peritoneovenous shunt for treatment of refractory ascites.
J Vasc Interv Radiol. 19(12):1717-22, 2008
5- Rahman N, De Coppi P, Curry J, Drake D, Spitz L, Pierro A, Kiely E:
Persistent ascites can be effectively treated by peritoneovenous
shunts. J Pediatr Surg. 46(8): 315–319, 2011
6- Herman R, Kunisaki S, Molitor M, Gadepalli S, Hirschl R, Geiger J:
The use of peritoneal venous shunting for intractable neonatal ascites:
a short case series. J Pediatr Surg. 46(8):1651-4., 2011
Corpus Luteum Cyst
A corpus luteum cyst (CLC) is a
functional ovarian cyst very rarely found in adolescent girls. The cyst
develops when the corpus luteum fails to regress following the release
of the ovum. CLC may rupture about the time of menstruation and take up
to three months to disappear completely. This type of cyst occurs after
an egg has been released from a follicle. The follicle becomes a
secretory gland known as corpus luteum. Produces large quantity of
estrogen and progesterone in preparation for conception, but if
pregnancy does not occur it disappears. If it fills with fluid or blood
it will grow and create a cyst. The cyst might cause pain by way of
torsion, rupture or bleeding. Fertility drugs used to induce an
ovulation increase the risk of corpus luteum cyst development.
Symptomatic large ovarian cysts will need imaging, genetic marker
determination and surgery. Laparoscopy is becoming the favored approach
by most pediatric surgeons for the treatment of ovarian cysts with
benign imaging and labs characteristics. All surgical procedures for
ovarian cysts should spare functional ovary as much as is
technically possible. The management of symptomatic corpus luteum cysts
is ovarian cystectomy using the tissue sparing procedure of stripping
of the cyst. In cases of endometrioma cysts the amount of ovarian
tissue removed together with the cyst is significantly much greater
than with the nonendometriotic
cysts.
References:
1- Mayer JP, Bettolli M, Kolberg-Schwerdt A, Lempe M, Schlesinger F,
Hayek I, Schaarschmidt K: Laparoscopic approach to ovarian mass in
children and adolescents: already a standard in therapy. J Laparoendosc
Adv Surg Tech A. 19 Suppl 1:S111-5, 2009
2- Shapiro EY, Kaye JD, Palmer LS: Laparoscopic ovarian cystectomy in children. Urology. 73(3):526-8, 2009
3- Karpelowsky JS, Hei ER, Matthews K: Laparoscopic resection of benign
ovarian tumours in children with gonadal preservation. Pediatr Surg
Int. 25(3):251-4, 2009
4- Palmara V, Sturlese E, Romeo C, Arena F, De Dominici R, Villari D,
Impellizzeri P, Santoro G: Morphological study of the residual ovarian
tissue removed by laparoscopy or laparotomy in adolescents with benign
ovarian cysts. J Pediatr Surg. 47(3):577-80, 2012
5- Kirkham YA, Kives S: Ovarian cysts in adolescents: medical and
surgical management. Adolesc Med State Art Rev. 23(1):178-91, 2012
6- Tessiatore P, Guanà R, Mussa A, Lonati L, Sberveglieri M,
Ferrero L, Canavese F: When to operate on ovarian cysts in children? J
Pediatr Endocrinol Metab. 25(5-6):427-33, 2012
PSU Volume 41 NO 04 OCTOBER 2013
Thromboprophylaxis
Thromboprophylaxis is utilized to
prevent and reduce the incidence of hospital-acquired life-threatening
venous thromboembolism (VTE) events such as deep venous thrombosis and
pulmonary embolism. The incidence of VTE in children has increased in
tertiary care centers. The presence of a central venous catheter is the
most prevalent risk factor for VTE in pediatric patients. Risk factors
associated with VTE include acute conditions such as major lower
extremity orthopedic surgery, spinal cord injury, major trauma to lower
extremity, lower extremity central venous catheter, acute infection,
burns and pregnancy. Chronic conditions include obesity, estrogen
containing medication, inflammatory bowel disease, nephrotic syndrome,
known thrombophilia. Other risk factors include past history of
previous DVT/PE or family history of VTE in first degree relatives.
Adolescent above the 14 years of age with above risk factors should
receive prophylaxis. Intervention for thromboprophylaxis includes early
and frequents ambulation, good hydration for low risk children;
mechanical prophylaxis using graduated compression antiembolic
stockings or sequential pneumatic compression for moderate risk; and
anticoagulant prophylaxis with enoxaparin or fractionated heparin for
high risk patients. For immobile patient sequential compression is
preferred. Contraindications for anticoagulation include intracranial
hemorrhage, acute stroke, uncontrolled hemorrhage, coagulopathy,
incomplete spinal cord injury, allergy and heparin induced
thrombocytopenia. Every institution managing children at high risk
should institute an algorithm of risk assessment and prophylaxis to
prevent VTE. Providing thromboprophylaxis to children is cost-effective.
References:
1- Jackson PC, Morgan JM: Perioperative thromboprophylaxis in children:
development of a guideline for management. Paediatr Anaesth.
18(6):478-87, 2008
2- Monagle P, Chalmers E, Chan A, DeVeber G, Kirkham F, Massicotte P,
Michelson AD; American College of Chest Physicians.
Antithrombotic therapy in neonates and children: American College of
Chest Physicians Evidence-Based Clinical Practice Guidelines (8th
Edition). Chest. 133(6 Suppl):887S-968S, 2008
3- Raffini L, Trimarchi T, Beliveau J, Davis D: Thromboprophylaxis in a
Pediatric Hospital: A Patient-Safety and Quality-Imrpovement
Intitative.Pediatrics. 127(5):e1326-32, 2011
4- Parker RI: Thromboprophylaxis in critically ill children: how should
we define the "at risk" child? Crit Care Med. 39(7):1846-7, 2011
5- Hanson SJ, Punzalan RC, Arca MJ, Simpson P, Christensen MA, Hanson
SK, Yan K, Braun K, Havens PL: Effectiveness of clinical guidelines for
deep vein thrombosis prophylaxis in reducing the incidence of venous
thromboembolism in critically ill children after trauma. J Trauma Acute
Care Surg. 72(5):1292-7, 2012
6- Sharma M, Carpenter SL: Thromboprophylaxis in a pediatric hospital.
Curr Probl Pediatr Adolesc Health Care. 43(7):178-83, 2013
Mucopolysaccharidosis
Mucopolysaccharidosis (MPS) are a
group of metabolic disorders due to absence or malfunctioning of a
lysosomal enzyme needed to breakdown molecules called
glycosaminoglycans causing a storage lysosomal disease. Children with
MPS are at high-risk for significant perioperative mortality. Excessive
secretions, difficult or failed intubation, need for emergency
tracheotomy and intraoperative cardiac arrest have been described in
MPS patients. The most studied is MPS type I caused by a deficiency of
lysosomal enzyme alpha-L-iduronidase producing accumulation of dermatan
sulfate and heparan sulfate in the lysosomes. There is a spectrum of
clinical disease involvement depending on age of onset, progression,
cognitive involvement and organ involvement. Management of children
with MPS type I include hematopoietic stem cell transplantation and
recombinant human alpha-L-idurodinase enzyme replacement.
Disease-related airway issues have been shown to increase the risk of
transplant in MPS type I. Many deaths associated to MPS I are due to
upper airway obstruction encountered during anesthetic care specially
in children with the most severe phenotype. Numerous airway problems
have been reported, including obscured airway landmarks owing to excess
glycosaminoglycan deposition, copious thick secretions, narrow stiff
airways, and difficulty oxygenating owing to glycosaminoglycan
deposition within alveoli. Surgical mortality may be greater in these
undiagnosed patients who are unlikely to be referred to
anesthesiologists with expertise in managing difficult airways or to
undergo other precautionary measures. Physicians should become familiar
with the physical characteristics and surgical history that suggest MPS
disorders and refer such patients to geneticists for evaluation before
surgery.
References:
1- Arn P, Wraith JE, Underhill L: Characterization of surgical
procedures in patients with mucopolysaccharidosis type I: findings from
the MPS I Registry. J Pediatr. 154(6):859-64.e3, 2009
2- Munoz-Rojas MV, Bay L, Sanchez L, van Kuijck M, Ospina S, Cabello
JF, Martins AM: Clinical manifestations and treatment of
mucopolysaccharidosis type I patients in Latin America as compared with
the rest of the world. J Inherit Metab Dis. 34(5):1029-37, 2011
3- Osthaus WA, Harendza T, Witt LH, et al: Paediatric airway management
in mucopolysaccharidosis 1: a retrospective case review. Eur J
Anaesthesiol. 29(4):204-7, 2012
4- Arn P, Whitley C, Wraith JE, Webb HW, Underhill L, Rangachari L, Cox
GF: High rate of postoperative mortality in patients with
mucopolysaccharidosis I: findings from the MPS I Registry. J Pediatr
Surg. 47(3):477-84, 2012
5- Kirkpatrick K, Ellwood J, Walker RW: Mucopolysaccharidosis type I
(Hurler syndrome) and anesthesia: the impact of bone marrow
transplantation, enzyme replacement therapy, and fiberoptic intubation
on airway management. Paediatr Anaesth. 22(8):745-51, 2012
6- Leboulanger N, Louis B, Fauroux B: The acoustic reflection method
for the assessment of paediatric upper airways. Paediatr Respir Rev.
2013 May 13
Trichilemmal Cyst
Cystic lesions of the skin in children
are fairly common. Most cases are either sebaceous or pilomatrixoma
cysts. Cysts where keratinization occurs without keratohyaline granules
derived from the follicular isthmus of the external root sheath of the
hair follicle are called trichilemmal or pillar cysts. Trichilemmal
cysts occur most commonly in the scalp due to the dense hair follicle
concentration. Face, trunks, back and forehead are the other common
site in that order. Trichilemmal cysts can occur as sporadic lesions or
in hereditary-familial settings with autosomal dominant transmission.
Though almost always benign, malignant transformation can occur rarely.
They may be locally aggressive becoming large and ulcerated.
Proliferating trichilemmal tumor is a solid cystic neoplasm that shows
differentiation similar to that of the isthmus of the hair follicle.
Trichilemmal cysts are usually a solitary intradermal or subcutaneous
lesions. The cyst is lined by stratified squamous epithelium.
They can grow to large sizes. Management of trichilemmal cyst consists
of surgical excision. The cytologic diagnosis of pilar cysts is
important because these cysts recur if incompletely excised and often
undergo transformation to pilar tumors.
References:
1- Adachi N, Yamashita T, Ito H: Differential diagnosis of scalp trichilemmal cyst on MRI. Dermatology. 193(3):263-5, 1996
2- Shet T, Rege J, Naik L: Cytodiagnosis of simple and proliferating trichilemmal cysts. Acta Cytol. 45(4):582-8, 2001
3- Nakamura M, Toyoda M, Kagoura M, Higaki S, Morohashi M:
Ultrastructural characteristics of trichilemmal cysts: report of two
cases. Med Electron Microsc. 34(2):134-41, 2001
4- Garg PK, Dangi A, Khurana N, Hadke NS: Malignant proliferating
trichilemmal cyst: a case report with review of literature. Malays J
Pathol. 31(1):71-6, 2009
5- Ibrahim AE, Barikian A, Janom H, Kaddoura I: Numerous recurrent
trichilemmal cysts of the scalp: differential diagnosis and surgical
management. J Craniofac Surg. 23(2):e164-8, 2012
6- Seidenari S, Pellacani G, Nasti S, Tomasi A, Pastorino L, Ghiorzo P,
Ruini C, Bianchi-Scarrà G, Pollio A, Mandel VD, Ponti G:
Hereditary trichilemmal cysts: a proposal for the assessment of
diagnostic clinical criteria. Clin Genet. 84(1):65-9, 2013
PSU Volume 41 NO 06 NOVEMBER 2013
Atrophic Testis
Atrophic testes refer to a testis that has diminished in size
and is accompanied by loss of function. An atrophic testis can be
the result of perinatal torsion, cryptorchidism, trauma, previous
surgical procedure, orchitis and steroid use. Most atrophic testes are
abnormal due to a mechanical event shunting circulation rather than
maldevelopment or iatrogenic. Anabolic steroids can cause testicular
atrophy by reducing the amount of luteinizing hormone produced by the
pituitary gland. Repair of inguinal hernia after incarceration can also
cause testicular atrophy if vascular testicular occlusion occurred for
en extended period of time. Testicular atrophy can occur in almost 50%
of cases of non-palpable undescended testes. During perinatal descent
the testis circulation is entrapped causing the atrophy or vanishing of
the testis. They have sometimes been called vanishing testes
since a remnant nubbin is found during inguinal or abdominal
exploration. Very few of these remnants contains seminiferous tubules
and even fewer shows viable germ cells. Contralateral testicular
hypertrophy strongly indicates an atrophic testis in the other side.
Ultrasonography can determine the significant smaller size of the
affected testis and determine if is hypoechoic, a sonographic
characteristic of the atrophic testis. The management of the atrophic
testis is controversial. Removal of the remnant and placement of a
prothesis is an alternative in children. With growth this prothesis
will need replacement, hence placement during final adolescent growth
years is more prudent. The risk of malignant degeneration of the
testicular remnant is extremely low to justify surgical removal.
References:
1- Wood HM, Elder JS: Cryptorchidism and Testicular Cancer: Separating Fract from Fiction. J Uro 181 (2): 452-461, 2009
2- Antic T, Hyjek EM, Taxy JB: The Vanishing Testis. A
histomorphologic and clinical assessment. AM J Clin Pathol. 136:
872-880, 2011
3- Cortes D, Thorup J, Petersen BL: Testicular neoplasia in
undescended testes of cryptorchid boys-does surgical strategy have an
impact on the risk of invasive testicular neoplasia? Turk J Pediatr. 46
Suppl:35-42, 2004
4- Chu L, Averch TD, Jackman SV: Testicular infarction as a sequela of inguinal hernia repair. Can J Urol. 16(6):4953-4, 2009
5- Shibata Y, Kojima Y, Mizuno K, Nakane A, Kato T, Kamisawa H,
Kohri K, Hayashi Y: Optimal cutoff value of contralateral testicular
size for prediction of absent testis in Japanese boys with nonpalpable
testis. Urology. 76(1):78-81, 2010
6- Vijayaraghavan SB: Sonographic localization of
nonpalpable testis: Tracking the cord technique. Indian J Radiol
Imaging. 21(2):134-41, 2011
Tension Gastrothorax
The term tension gastrothorax originally appeared in the
literature as a complication of traumatic rupture of the diaphragm
producing mediastinal shift due to a distended intrathoracic stomach.
Congenital or acquired diaphragmatic defects can cause a tension
pneumothorax. The two groups of children that can be affected by a
tension gastrothorax include those with congenital diaphragmatic hernia
with late presentation and traumatic diaphragmatic hernias the result
of a previous accident. Most cases of tension gastrothorax occur around
the five years of age in cases with an existing congenital
defect. Vast majority are left-sided because the liver buttresses the
right side. Increased intraabdominal pressure or negative intrathoracic
pressure leads to herniation of the stomach into the chest. Respiratory
symptoms initially followed by abdominal pain and vomiting develops.
Other findings are tracheal deviation, reduced breaths sound, dullness
or resonance and a displace cardiac apex. Tension gastrothorax can be
erroneously interpreted as a tension pneumothorax leading to increase
morbidity and mortality during treatment. The chest film can be
diagnostic demonstrating a large air-filled structure with or without a
fluid level within the left hemithorax causing apical collapse of the
lung. Emergency management requires initial decompression with a
large-bore nasogastric tube. If this fails transthoracic needle
decompression of the stomach can be tried. Urgent definitive management
requires surgical reduction of the intrathoracic stomach and repair of
the diaphragmatic defect which can be accomplished preferably by
laparotomy as it hasten quick reduction of stomach and repair of the
diaphragmatic defect. Thoracotomy or thoracoscopy has also been
utilized less frequently. Morbidity relates to pulmonary collapse,
shock, bowel injury and sepsis due to gastric perforation in the thorax.
References:
1- Rathinam S, Margabanthu G, Jothivel G, Bavanisanker T:
Tension gastrothorax causing cardiac arrest in a child. Interact
Cardiovasc Thorac Surg. 1(2):99-101, 2002
2- Zedan M, El-Ghazaly M, Fouda A, El-Bayoumi M: Tension gastrothorax:
a case report and review of literature. J Pediatr Surg. 43(4):740-3,
2008
3- Salim F, Ramesh V: Tension gastrothorax: a rare complication. J Coll Physicians Surg Pak. 19(5):325-6, 2009
4-Hooker R, Claudius I, Truong A: Tension gastrothorax in a child
presenting with abdominal pain. West J Emerg Med. 13(1):117-8, 2012
5- Gagg JW, Savva A: Tension gastrothorax: a rare cause of breathlessness. Emerg Med J. 30(6):500, 2013
6- Ng J, Rex D, Sudhakaran N, Okoye B, Mukhtar Z: Tension gastrothorax
in children: Introducing a management algorithm. J Pediatr Surg.
48(7):1613-7, 2013
Adrenalectomy in Wilms Tumor
Wilms tumor or nephroblastoma is the most common
intraabdominal malignant solid tumor in children. It is managed with
surgery, adjuvant chemotherapy and radiotherapy. Surgical management
consists of radical nephrectomy when appropriate in many cases removing
the adrenal gland concomitantly with the tumor. Adrenal involvement in
patients with Wilms tumor is rare and difficult to predict. The
decision to remove the ipsilateral adrenal gland has been left to the
judgement of the operating surgeon at the time of nephrectomy, and is
likely based on the size and location of the primary tumor, ease of
adrenalectomy, and suspicion for adrenal involvement Routine
adrenalectomy does not confer a benefit for oncologic control (event
free survival) when it is feasible to spare the adrenal gland.
Intraoperative tumor spillage rates are higher in patients undergoing
concomitant adrenalectomy. Patients in whom adrenalectomy was performed
tended to have larger tumors than those in whom the gland was left in
situ. The histopathologic status of the adrenal gland with tumor does
not directly affect the oncologic outcome. Based on the low rate of
adrenal involvement, and lack of apparent oncologic benefit to
adrenalectomy concurrent with nephrectomy routine adrenalectomy does
not appear to be mandatory. Preserving the adrenal gland was not
associated with an increased risk of local recurrence. The above
appears to hold truth to renal cell carcinoma management in adults.
References:
1- Yap SA, Alibhai SM, Abouassaly R, Timilshina N, Finelli A: Do we
continue to unnecessarily perform ipsilateral adrenalectomy at the time
of radical nephrectomy? A population based study. J Urol.
187(2):398-404, 2012
2- Gow KW, Barnhart DC, Hamilton TE, Kandel JJ, Chen MK, Ferrer FA,
Price MR, Mullen EA, Geller JI, Gratias EJ, Rosen N, Khanna G, Naranjo
A, Ritchey ML, Grundy PE, Dome JS, Ehrlich PF: Primary nephrectomy and
intraoperative tumor spill: report from the Children's Oncology Group
(COG) renal tumors committee. J Pediatr Surg. 48(1):34-8, 2013
3- Tsui KH, Shvarts O, Barbaric Z, Figlin R, de Kernion JB, Belldegrun
A: Is adrenalectomy a necessary component of radical nephrectomy? UCLA
experience with 511 radical nephrectomies. J Urol. 163(2):437-41,
2000
4- Moore K, Leslie B, Salle JL, Braga LH, Bägli DJ,
Bolduc S, Lorenzo AJ: Can we spare removing the adrenal gland at
radical nephrectomy in children with wilms tumor? J Urol. 184(4
Suppl):1638-43, 2010
5- Kieran K, Anderson JR, Dome JS, Ehrlich PF, Ritchey ML, Shamberger
RC, Perlman EJ, Green DM, Davidoff AM: Is adrenalectomy necessary
during unilateral nephrectomy for Wilms Tumor? A report from the
Children's Oncology Group. J Pediatr Surg. 48(7):1598-603, 2013
6- Green DM: The evolution of treatment for Wilms tumor. J Pediatr Surg. (48): 14-19, 2013
PSU Volume 41 No 06 DECEMBER 2013
Granular Cell Tumor
Granular cell tumor, also
known as Abrikossoff tumor, is a very infrequent benign neoplasm
affecting all parts of the body, but with a predilection for the head
and neck region. In the head and neck region most cases are localized
in the oral cavity, especially the tongue. Granular cell tumor (GCT) is
twice as common in females as in males. These tumors usually present as
a solitary slow-growing ulcerated nodular mass located mainly in the
subcutaneous tissue. The lesion is mobile and not painful with mild
pruritus. Multiple development of granular cell tumor can be seen
associated with Noonan syndrome and Neurofibromatosis. In children, the
most frequent presentation of GCT is congenital epulis, arising from
the median ridge of the newborn maxilla. The histogenesis of the tumor
is from neural or nerve sheath cells. Histopathology of the tumor shows
polygonal cells arranged in sheets with granular eosinophil cytoplasm
and small nuclei. The granularity of the tumor cells is due to
accumulation of secondary lysosome in the cytoplasm of the cells. An
aggressive malignant variant of granular cell tumor is extremely rare.
There are no well-defined criteria for the diagnosis of malignancy.
Tumor size > 5 cm, rapid growth, vascular invasion, necrosis and
cell spindling are important indicators of atypia pertaining
malignancy. Occurrence of metastasis is the only accepted criteria of
malignancy. Malignancy when present is associated with a poor
prognosis. Management of granular cell tumor consists of complete
surgical excision. Recurrence is uncommon unless surgical excision has
been incomplete. Follow-up must be prolonged.
References:
1- Finck C, Moront M, Newton C, Timmapuri S, Lyons J, Rozans M, de
Chadarevian JP, Halligan G: Pediatric granular cell tumor of the
tracheobronchial tree. J Pediatr Surg. 43(3):568-70, 2008 2- Dupuis C,
Coard KC: A review of granular cell tumours at the University Hospital
of the West Indies: 1965-2006. West Indian Med J. 58(2):138-41,
2009
3- Ramaswamy PV, Storm CA, Filiano JJ, Dinulos JG: Multiple granular
cell tumors in a child with Noonan syndrome. Pediatr Dermatol.
27(2):209-11, 2010
4- Torre M, Yankovic F, Herrera O, Borel C, Latorre JJ, Aguilar P,
Varela P: Granular cell tumor mimicking a subglottic hemangioma. J
Pediatr Surg. 45(12):e9-11, 2010
5- Nasser H, Ahmed Y, Szpunar SM, Kowalski PJ: Malignant granular cell
tumor: a look into the diagnostic criteria. Pathol Res Pract.
207(3):164-8, 2011
6- Lahmam Bennani Z, Boussofara L, Saidi W, Bayou F, Ghariani N,
Belajouza C, Sriha B, Denguezli M, Nouira R: [Childhood cutaneous
Abrikossoff tumor]. Arch Pediatr. 18(7):778-82, 2011
Watersport Injuries
Injuries related to personal watercraft have increased
dramatically over the past several years, becoming one of the leading
causes of recreational watersport injuries. Median age of the accident
is generally 10 years. Towed tubing which involves riding an inflatable
tube while being pulled behind a boat is the most prevalent trauma
mechanism in watersport injury. This is followed by accidents involving
motorboats and accidents involving a personal watercraft. Towed tubing
accidents has a longer hospital stay since this mechanism account for
an increased morbidity in comparison with other recreational
activities. It is strongly suggested that wearing protective gear and
protective wet suit should be recommended for children involved in
watercraft and watersport activities. Mandatory speed limit regulations
should be considered to decrease the risk of serious injury due to the
specific category of the watercraft injury. Propeller injuries can
produce an increase incidence of distal limb amputations. Almost 20% of
all boating fatalities and 8% of all non-fatal injuries are associated
with accidents in which alcohol or drugs were contributing factors. Jet
ski accidents tended to result in more serious injuries (closed-head
injuries, hollow and solid viscus injuries, chest trauma, spinal
injuries leading to paralysis, and death) than those sustained in
accidents with small boats. It is also recommended life vest and easily
visible personal floating devices be used by children and adolescent
riding watercrafts. Increased use of personal floating device,
avoidance of dangerous currents, and less alcohol use by operators and
passengers of all types of watercraft would result in a reduction in
watercraft-related drowning. Government statistics on personal
watercraft injuries do not accurately reflect the true incidence and
economic impact of such trauma. Mandatory educational programs and
increased legislation to improve personal watercraft safety should be
promoted.
References:
1- Browne ML, Lewis-Michl EL, Stark AD: Watercraft-related drownings
among New York State residents, 1988-1994. Public Health Rep.
118(5):459-63, 2003
2- Kim CW, Smith JM, Lee A, Hoyt DB, Kennedy F, Newton PO, Meyer RS:
Personal watercraft injuries: 62 patients admitted to the San Diego
County trauma services. J Orthop Trauma. 17(8):571-3, 2003
3- Rubin LE, Stein PB, DiScala C, Grottkau BE: Pediatric trauma caused
by personal watercraft: a ten-year retrospective. J Pediatr Surg.
38(10):1525-9, 2003
4- White MW, Cheatham ML: The underestimated impact of personal watercraft injuries. Am Surg. 65(9):865-9, 1999
5- Beierle EA, Chen MK, Langham MR Jr, Kays DW, Talbert JL: Small watercraft injuries in children. Am Surg. 68(6):535-8, 2002
6- Keijzer R, Smith GF, Georgeson KE, Muensterer OJ: Watercraft and
watersport injuries in children: trauma mechanisms and proposed
prevention strategies. J Pediatr Surg. 48(8):1757-61, 2013
Stuck Catheter
Totally implantable venous access devices are essential for
providing therapy for children with cancer, long-term medication,
parenteral nutrition and sampling. Unfortunately they are no without
complications during insertion, maintenance and removal. Removal occurs
with resolution of disease, dysfunction or infection of the device. One
of the most feared complications during removal is the stuck catheter.
The catheter after a prolonged period of use is fixed to the vessel
wall. The catheter is stuck in strong connective fibrous tissue matrix
to the vessel wall. This occurs more commonly with polyurethane
catheter than silicone catheters. In fact polyurethane catheters are
contraindicated if its going to be in use for more than 18 months. The
management of a stuck catheter should follow a course of action
encompassing dissection along the subcutaneous tract until the entrance
to the vein is encountered. A stiff guide wire can be inserted into the
lumen of the catheter and instead of applying a "pull-out" force, use a
"push-in" force to detach the catheter from the deep central vein. When
the forced "pull-out" maneuver is used, the catheter can stretch and
will likely break if the tension exceeds the tolerance of the catheter.
The soft J tip of the guide-wire prevents puncture of the catheter or
the wall of the heart and vein during the maneuver In other occasion
the catheter will brake during removal and either stay stuck in the
vein or embolize. In such situation endovascular retrieval by
interventional radiologist of the segment of left catheter is indicated
due to the inherent risk of infection and venous thrombosis. Have found
useful after passing the wire to the stuck catheter cleaning the
adherence of fibrin reintroducing a new sheath through the catheter
fluoroscopically-guided.
References:
1- Wilson GJ, van Noesel MM, Hop WC, van de Ven C: The catheter is
stuck: complications experienced during removal of a totally
implantable venous access device. A single-center study in 200
children. J Pediatr Surg. 41(10):1694-8, 2006
2- Field M, Pugh J, Asquith J, Davies S, Pherwani AD: A stuck
hemodialysis central venous catheter. J Vasc Access. 9(4):301-3, 2008
3- Huang SC, Tsai MS, Lai HS: A new technique to remove a "stuck"
totally implantable venous access catheter. J Pediatr Surg.
44(7):1465-7, 2009
4- Mortensen A, Afshari A, Henneberg SW, Hansen MA: Stuck long-term
indwelling central venous catheters in adolescents: three cases and a
short topical review. Acta Anaesthesiol Scand. 54(6):777-80, 2010
5- Hong JH: A breakthrough technique for the removal of a hemodialysis
catheter stuck in the central vein: endoluminal balloon
dilatation of the stuck catheter. J Vasc Access. 12(4):381-4, 2011
6- Ryan SE, Hadziomerovic A, Aquino J, Cunningham I, O'Kelly K, Rasuli
P: Endoluminal dilation technique to remove "stuck" tunneled
hemodialysis catheters. J Vasc Interv Radiol. 23(8):1089-93, 2012

