PSU Volume 56 NO 01 JANUARY 2021
Central Venous Catheter Tip Placement
Central venous catheters (CVC) are essential for providing
long-term chemotherapy for cancer, total parenteral nutrition, central
venous pressure monitoring, secure regular blood sampling, and
prolonged intravenous management in children. Using ultrasound and
Seldinger technique, the internal jugular, external jugular, cephalic
or subclavian vein are usually cannulated as entrance points. The
anatomic position of the tip of the CVC is of upmost importance
to avoid dysrhythmias, thrombosis, valvular insufficiency, inaccurate
venous pressure monitoring, tricuspid valve damage, and perforation of
the right atrium (RA), right ventricle or superior vena cava (SVC)
resulting in cardiac tamponade. Tips located outside the heart are
associated with thrombosis of the cava or its tributaries, inadvertent
infusion into nontarget vessels, catheter dysfunction, erosion of the
catheter into the lung or bronchus and risk of perforation with
hemothorax. Catheter angulation, curvature or looping is a major risk
for perforation. The recommended position of the tip of the CVC is just
above the superior vena cava-right atrial junction parallel to the
SVC to prevent serious complications. Radiographic and
fluoroscopy methods are the standard for defining tip position follow
very closely by ultrasound and EKG. Several radiographic landmarks have
been used to determine the exact position of the tip of the catheter in
the SVC-RA junction. These include the right tracheobronchial angle and
the carina. The right tracheobronchial angle has less clinical
applicability as radiologist find difficult to identify in some chest
films. The carina has several advantages as radiologic landmark of the
catheter tip: it does not move with pathologic changes in the lung, it
is positioned in the center of the body and it can be identified easily
even in poor quality chest films. In adolescent and young adults a
point approximately two vertebral body units below the carina was found
as the landmark of the cavoatrial junction. In order to place the
catheter tip at the level of the carina several external landmarks must
be used to cut the proper length of catheter during insertion. The CVC
tip can be reliably placed near the carina level using the external
landmarks of the sternal head of the right clavicle and a perpendicular
line connecting both nipples. The catheter distance should be measured
from the insertion point to midpoint the distance between these two
landmarks substrated by 0.5 cm and cut. The insertion depth is
determined placing the CVC over the sterilized skin from the insertion
point to the midpoint of the perpendicular line joining the sternal
head of the right clavicle and the line connecting both nipples.
Alternatively, the depth of insertion of the catheter can be determined
by the distance from the skin puncture to the second intercostal space
(sternal angle of Lewis). The length of the CVC using either the carina
or SVC-RA junction as measured by thoracic CT-Scan correlates with the
patient age and body surface area and formulas for each catheter length
have been devised previously. The carina is still an easily sighted and
clear radiological landmark in children to confirm that the CVC tip is
outside the pericardial reflection. In neonates the carina is not
always located above the pericardium, therefore, the carina could not
be an appropriate landmark for CVC placement. In all cases, a chest
film is mandatory after CVC placement to determine tip position and
associated complications of punctured.
References:
1- Andropoulos DB, Bent ST, Skjonsby B, Stayer SA: The Optima Length of
Insertion of Central Venous Catheters for Pediatric Patients. Anesth
Analg. 93: 883-886, 2001
2- Yoon SZ, Shin JH, Hahn S, et al: Usefulness of the carina as a
radiographic landmark for central venous catheter placement in
paediatric patients. Br J Anaesth. 95(4): 514-517, 2005
3- Baskin KM, Jimenez RM, Cahill AM, Jawad AF, Towbin RB: Cavoatrial
Junction and Central Venous Anatomy: Implications for Central Venous
Access Tip Position. J vasc Interv Radiol 19: 359-365, 2008
4- Na HS, Kim JT, Kim HS, et al: Practical anatomic landmarks for
determining the insertion depth of central venous catheter in
paediatric patients. Br J Anaesth. 102(6): 820-823, 2009
5-Witthayapraphakorn L, Khositseth A, Jiraviwatana T, et al:
Appropriate Length and Position of the Central Venous Catheter
Insertion via Right Internal Jugular Vein in Children. Indian Pediatr.
50:749-752, 2013
6- Perin G, Scarpa MG: Defining central venous line position in children: tips for the tip. J Vasc Access. 16(2): 77-86, 2015
7- Hoffmann S, Goedeke J, Konig TT, et al: Multivariate analysis on
complications of central venous access devices in children with cancer
and severe disease influenced by catheter tip position and vessel
insertion site (A STROBE-compliant study). Surgical Oncology. 34:
17-23, 2020
8- Maddali MM, Al-Shamsi F, Arora NR, Panchatcharam SM: The Optimal
Length of Insertion for Central Venous Catheters Via the Right Internal
Jugular Vein in Pediatric Cardiac Surgical Patients. J Cardiothoracic
and Vasc Anesth. 34: 2386-2391, 2020
Bilateral Papillary Thyroid Carcinoma
Of all thyroid cancers occurring in the USA, around 10%
occurs during the pediatric age. Almost 20% of all solid thyroid
nodules in children harbor a malignancy. The most common histological
type of thyroid malignancy found in children is papillary carcinoma,
which together with the less common follicular variety (< 5%)
comprise most cases of differentiated thyroid cancer in children.
Differentiated thyroid carcinoma arises from the thyroid follicular
cells. Papillary thyroid carcinoma (PTC) in children is characterized
by a high incidence of regional lymph node metastasis at the time of
diagnosis (60%), along with a higher incidence of bilateral and
multifocal disease (30% and 65% respectively. In spite of this,
children are less likely to die from disease, with disease-specific
mortality less than 3%. The three most common risk factors identified
in children with PTC include exposure to ionizing radiation in the head
and neck area, a family history of thyroid cancer and a preoperative
diagnosis of Hashimoto thyroiditis. A variety of genetic disorders may
predispose to PTC including familial adenomatoid-polyposis, Carney
complex, Werner syndrome, DICER1 syndrome and hamartoma tumor syndrome.
The presence of lateral neck lymph node metastasis noted in
preoperative ultrasound studies is associated with an increase risk of
detectable bilateral PTC. FNA should be performed on any suspicious
lymph nodes in the lateral neck as confirmation of metastatic
involvement prior to lateral neck dissection. Of children diagnosed
preoperatively with unilateral disease, if the dominant tumor measured
greater than 2 cm a postoperative diagnosis of bilateral disease is
more likely. Children with occult bilateral disease are more likely to
have positive central compartment lymph node involvement, extranodal
extension of disease, extrathyroidal extension, lymphovascular invasion
and multifocal disease. Diffuse-sclerosing variant tumors is associated
with an increase of bilateral disease. Total thyroidectomy maximizes
disease-free survival, overall survival and quality-adjusted life
expectancy in children with PTC compared with lobectomy. Almost
one-fourth of children have occult contralateral disease with a high
risk for persistent disease if a total thyroidectomy is not performed
at the time of diagnosis. Lesser thyroid resection than total
thyroidectomy is associated with as high as 10-fold greater recurrences
rates. Inadequate lymph node dissections in patients with clinically
positive nodes increase the need for subsequent intervention 3-fold.
Addition of central lymph node dissection to total thyroidectomy in PTC
decrease recurrence rate to less than 5%at ten
years.
References:
1- Baumgarten H, Jenks CM, Isaza A, et al: Bilateral papillary thyroid
cancer in children: Risk factors and frequency of postoperative
diagnosis. J Pediatr Surg. 55(6):1117-1122, 2020
2- Christison-Lagay ER, Beartschiger RM, Dinauer C, et al: Pediatric
differentiated thyroid carcinoma: An update from the APSA Cancer
Committee. J Pediatr Surg.http://doi.org/10.1016/j.pedsurg2020.05.003,
2020
3- Hay ID, Johnson TR, Kaggal S, et al: Papillary Thyroid Carcinoma
(PTC) in Children and Adults: Comparison of Initial Presentation and
Long-Term Postoperative Outcome in 4432 Patients Consecutively Treated
at the Mayo Clinic During Eight Decades (1936-2015). World J Surg.
42(2):329-342, 2018
4- Qu N, Zhang L, Wu WL, et al: Bilaterality weighs more than
unilateral multifocality in predicting prognosis in papillary thyroid
cancer. Tumour Biol. 37(7):8783-9, 2016
5- Lee YS, Lim YS, Lee JC, et al: Clinical implications of bilateral
lateral cervical lymph node metastasis in papillary thyroid cancer: a
risk factor for lung metastasis. Ann Surg Oncol. 18(12):3486-92, 2011
Zenker Diverticulum
Zenker diverticulum is considered the most common
diverticulum of the esophagus. It is usually seen in the 6th to 8th
decade of life with only a few cases described in the pediatric age.
Zenker diverticulum is a protrusion of pharyngo-esophageal mucosa
through a weak zone in the posterior wall of the pharynx known as
Killian's triangle. It is an acquired hernia of the posterior
pharyngeal mucosa membrane at the pharyngo-esophageal junction
occurring between fibers of the lower pharyngeal constrictor and
crico-pharyngeal muscles. Dysfunction of the cricopharyngeal muscle
plays a major role in the pathogenesis of the diverticulum. It is a
pulsion pseudodiverticulum since is associated with high intraluminal
pressure and does not contain all the layers of the esophagus. Being a
pseudodiverticulum there is involvement of only the mucosal layer. They
are more common in males and present more frequently on the left side.
Zenker diverticulum causes dysphagia, a sensation of food sticking in
the throat, noisy deglutition, regurgitation of undigested food, cough,
aspiration, chronic foreign body impaction and halitosis. Over time
patients may present weight loss due to chronic dysphagia. Esophagogram
(barium swallow is the gold standard), US or CT-Scan with oral contrast
of the neck and proximal thorax are diagnostic, revealing a
diverticular pouch filled with air, debris and food particles
compressing the trachea. Zenker diverticulum is classified by their
longitudinal size into small (diameter less than 2 cm), medium
(diameter 2-4 cm), and large (diameter > 4 cm). Zenker diverticulum
is associated with Marfan syndrome due to the abnormal weak wall
related to the connective tissue disorder. The differential diagnosis
includes a congenital crico-pharyngeal diverticulum, duplication of the
esophagus, traction diverticulum, a false diverticulum above a
congenital stenosis, traumatic pseudodiverticulum of the pharynx in
newborns (perforation by nasogastric tube), and a postoperative
diverticulum after repair of tracheo-esophageal fistula. Management of
a Zenker diverticulum depends on the location, symptoms and size of the
diverticulum. Endoscopy stapling or laser resection, diverticulectomy,
cricopharyngeal myotomy or diverticulopexy are several procedures
performed for Zenker diverticulum. Myotomy is the mainstay of treatment
with favorable outcomes in more than 80% of patients with a reduced
recurrence rate. Endoscopic management is not recommended in large
diverticula because of incomplete emptying of pouch
remnants.
References:
1- Gorkem SB, Yikilmaz A, Coskun A, Kucukaydin M: A pediatric case of
Zenker diverticulum: imaging findings. Diagn Interv Radiol 15: 207-209,
2009
2- Patron V, Godey B, Aubry K, Jegoux F: Case report. Endoscopic
treatment of pharyngo-esophageal diverticulum in child. Internat J
Pediatr Otorhinolaryng. 74: 694-697, 2010
3- Abu-Omar A, Miller C, McDermott AL: Acquired pharyngoesophageal
diverticulum in childhood. J Laryng & Otology. 124: 1298-1299, 2010
4- Lindholm EB, Hansbourgh F, Upp Jr JR, Cilloniz R, Lopoo J:
Congenital esophageal diverticulum - A case report and review of
literature. J Pediatr Surg. 48: 665-668, 2013
5- Bergeron JL, Chheri DK: Indications and Outcomes of Endoscopic CO2
Laser cricopharyngeal Myotomy. Larynsgoscope 124(4): 950-954, 2014
6- Crawley B, Dehom S, Tamares S, et al: Adverse Events after Rigid and
Flexible Endoscopic Repair of Zenker's Diverticula: A Systematic Review
and Meta-analysis. Otolaryngol Head Neck Surg. 161(3):388-400, 2019
PSU Volume 56 NO 02 FEBRUARY 2021
Congenital Short Bowel Syndrome
Short bowel syndrome is a clinical disorder characterized by
diarrhea, malabsorption and needs of parenteral nutrition for life
support. In newborns, short bowel syndrome is most commonly an acquired
disorder after surgical bowel resection due to conditions such as
necrotizing enterocolitis, gastroschisis, volvulus, extensive
aganglionosis in Hirschsprung's disease, or intestinal atresia. Born
with a short bowel known as congenital short bowel syndrome (CSBS) is a
very rare condition in newborns associated with a high mortality rate
and prognosis. According to autopsy reports, the length of the
small intestine as measured from Treitz to ileocecal valve correlates
with crown-to-heel length. The mean length of the small bowel in full
term infants is approximately 240 cm and increases to 600 cm in
adulthood. Short bowel syndrome manifests in newborns when the small
bowel length is less than 75 cm. Newborns with CSBS can be as short as
20 cm in length, with a mean length of 50 cm. The pathogenesis of CSBS
is poorly understood. CSBS occurs most often in association with
malrotation (> 96%). The normal elongation, rotation and herniation
of the small bowel is interrupted or delayed due to lack of space
between the developing digestive tube and umbilical celom. Other
believe is a defective neuroenteric development since intestinal
dysmotility is an important component of the syndrome. Children born
with CSBS have a loss of function nonsense mutation in the CLMP
(Coxsackie- and adenovirus receptor-like membrane protein). CLMP
encodes a tight-junction membrane protein of the bowel, located in
chromosome 11, and expressed during embryonic development. Mutations in
CLMP cause a recessive form of CSBS. In other patients a mutation in
FLNA gene was found in chromosome X, which encodes for a cytoskeletal
protein called filamin A that binds to actin. Other congenital
anomalies associated with CSBS include pyloric stenosis, appendiceal
agenesis, acheiria, dextrocardia, hemivertebrae and paten ductus
arteriosus. Clinically, most babies with CSBS develop bilious vomiting,
diarrhea, failure to thrive with signs/symptoms consistent with
intestinal obstruction. Onset of intestinal volvulus associated with
acute mesenteric ischemia is rare in these patients since a short bowel
length precludes twisting. CSBS is diagnosed by barium-contrast studies
and confirmed by exploratory laparotomy. Management of CSBS consist of
parenteral nutrition with early introduction of enteral nutrition. In
the event of failed adaptation and liver failure with reduced venous
access from treatment, transplantation becomes the hope for these
children. Survival beyond the first year with CSBS is 75% and
improving. Most common cause of death is
sepsis.
References:
1- Hasosah M, Lemberg DA, Skarsgard E, Schreiber R. Congenital short
bowel syndrome: a case report and review of the literature. Can J
Gastroenterol. 22(1):71-4, 2008
2- Van Der Werf C, Wabbersen TD, Hsiao NH, et al: CLMP is Required for
Intestinal Development, and Loss-of-Function Mutations Cause Congenital
Short-Bowel Syndrome. Gastroenterology 142: 453-462, 2012
3- Coletta R, Khalil BA, Morabito A. Short bowel syndrome in children:
surgical and medical perspectives. Semin Pediatr Surg. 23(5):291-7, 2014
4- Gonnaud L, Alves MM, Cremillieux C, et al: Two new mutations of the
CLMP gene identified in a newborn presenting congenital short-bowel
syndrome. Clinics and Research in Hepatology and gastroenterology 40:
e65-e67, 2016
5- Goulet O, Abi Nader E, Pigneur B, Lambe C. Short Bowel Syndrome as
the Leading Cause of Intestinal Failure in Early Life: Some Insights
into the Management. Pediatr Gastroenterol Hepatol Nutr. 22(4):303-329,
2019
6- Negri E, Coletta R, Morabito A. Congenital short bowel syndrome:
systematic review of a rare condition. J Pediatr Surg. 55(9):1809-1814,
2020
Biliary Dyskinesia
Biliary Dyskinesia (BD) is characterized by an abnormal
gallbladder contractility identified using cholecystokinin-stimulated
hepatobiliary iminodiacetic acid (HIDA) nuclear scans. BD is a
diagnosis of exclusion so other causes such as irritable bowel
syndrome, dyspepsia, GE reflux disease needs to be rule out. The
diagnosis is established when the gallbladder has an ejection fraction
less than 35% and the child has typical symptoms of biliary colic
without gallstones. Gallbladder dyskinesia is becoming the most common
indication for gallbladder removal in adults and pediatric population.
The most accepted pathogenesis of BD is uncoordinated contractions and
relaxation of both the gallbladder and sphincter of Oddi. Subsequent
distension of the gallbladder leads to inflammation, hypersensitivity
and dysfunction. 75% of affected cases are females, most cases
are white adolescents and almost 50% are obese. Major preoperative
symptoms in children with BD include nonspecific vague chronic right
upper quadrant/epigastric abdominal pain, nausea, postprandial pain,
fatty food intolerance, vomiting, constipation and diarrhea in this
order of frequency. Children with symptoms and BD undergo a series of
preoperative studies without significant pathologic findings such as
US, CT-Scan, MRI, UGIS and upper/lower GI endoscopy. Most of this cases
are referred by pediatric gastroenterologist after trying several
medical management options. Initially more than 80% claim pain
improvement after cholecystectomy, but two years or more after surgery
30-40% continue with similar symptoms. The difference between short and
long-term results may be due to true recurrence of symptoms or
inaccurate reporting by parents who wants to please the surgeon.
Two-third of gallbladders removed due to BD shows chronic
cholecystitis. Patients with chronic inflammation are more likely to
persist with symptoms at long-term follow-up. An ejection fraction
below 15% is usually associated with a higher resolution of symptoms
after cholecystectomy. BMI percent, pain during CCK administration
during the HIDA scan and presence of chronic cholecystitis does not
predict which patient will have short or long-term improvement in
symptoms. Factors independently associated with short term pain
improvement after cholecystectomy includes shorter duration of pain
before surgery, history of vomiting preop, no history of fevers or
obstructive sleep apnea. Factors independently associated with
short-term complete symptoms, resolution includes history of epigastric
pain and lower GI disease. Longer duration of symptoms predicts poor
outcome after cholecystectomy in BD. Symptoms of functional dyspepsia
overlap with symptoms of BD in children. There is no well-defined
nonsurgical management of BD. Some researchers advocate medical over
surgical management of BD since BD is not a life threatening condition,
there are no serious complications and some children continue to
experience symptoms after cholecystectomy. With persistent abdominal
pain after cholecystectomy there is a high index of suspicion for
sphincter of Oddi dysfunction. Studies comparing cholecystectomy to
nonsurgical medical management show same results in both
groups.
References:
1- Haricharan RN, Proklova LV, Aprahamian CJ, et al: Laparoscopic
cholecystectomy for biliary dyskinesia in children provides durable
symptoms relief. J Pediatr Surg. 43: 1060-64, 2008
2- Lacher M, Yannam GR, Muenterer OJ, et al: Laparoscopic
cholecystectomy for biliary dyskinesia in children: Frequency
increasing. J Pediatr Surg. 48: 1716-1721, 2013
3- Knott EM, Fike FB, Gasior AC, et al: Multi-institutional analysis of
long-term symptoms resolution after cholecystectomy for biliary
dyskinesia in children. Pediatr Surg Int 29: 1243-47, 2013
4- Mahida JB, Sulkowski JP, Cooper JN, et al: Prediction of symptoms
improvement in children with biliary dyskinesia. J Surg Research. 198:
393-399, 2015
5- Jones PM, Rosenman MB, Pfefferkorn MD, Rescorla FJ, Bennett Jr WE:
Gallbladde Ejection Fraction is Unrelated to Gallbladder Pathology in
Children and Adolescents. JPGN 63:71-75, 2016
6- Santuci NR, Hyman PE, Harmon CM, Schiavo JH, Hussain SZ: Biliary
Dyskinesia in Children: A Systematic Review. JPGN 64: 186-193, 2017
7- Coluccio M, Claffey AJ, Rothstein DH: Biliary Dyskinesia: Fat or
fiction?. Seminars Pediatr Surg.
Https://doi.org/10.1016/j.sempedsurg.2020.150947, 2020
8- Khan FA, Markwith N, Islam S: What is the role of the
cholecystokinin stimulated HIDA scan in evaluationg abdominal pain in
children?. J pediatr Surg. 55: 2653-2656, 2020
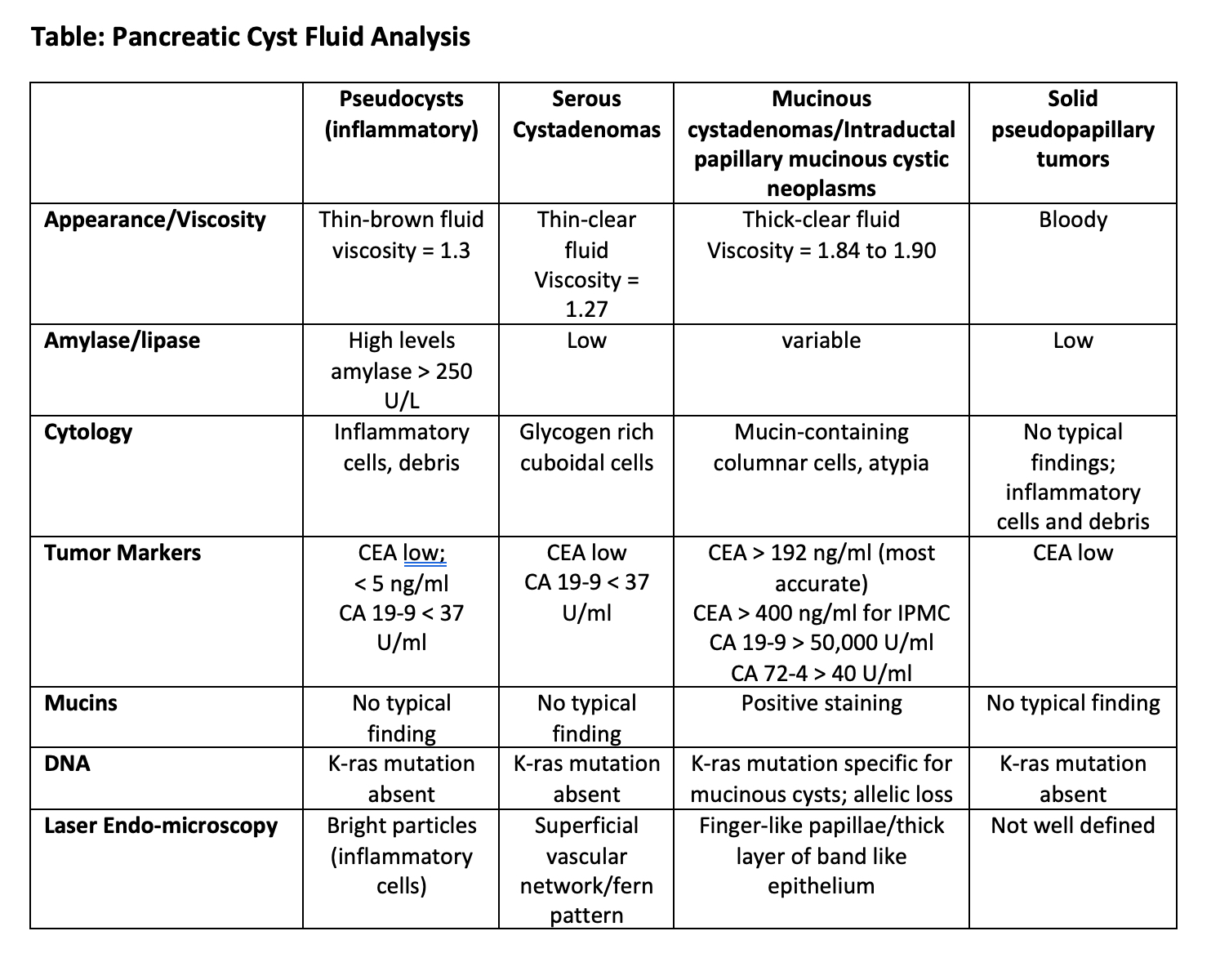
Pancreatic Cyst Fluid Analysis
The extensive use of ultrasound has uncovered
symptomatically as well as asymptomatic pancreatic cysts. The majority
of pancreatic cysts are found incidentally when abdominal imaging is
performed for other indications. Pancreatic cysts can either be simple
(retention) cysts, pseudocysts and cystic neoplasm. Cystic neoplasms
are further subdivided into serous cystadenomas, mucinous cystic
neoplasms, intraductal papillary mucinous neoplasms or papillary cystic
neoplasms. The majority of pancreatic cystic lesions identified are
mucinous cystic neoplasms. Since some of these pancreatic cysts can
develop into malignancy, a diagnosis performing aspiration and analysis
of the cyst fluid must be undertaken. Accurate diagnosis leads to
effective and standard management. This can be achieved using either
US- or CT-guided percutaneous aspiration or endoscopic ultrasound fine
needle aspiration. The diagnostic accuracy after aspiration is very
high (~95%). The fluid is analyzed for cytology, viscosity,
extracellular mucin, tumor markers (CEA, CA 19-9, CA 15-3, CA 72-4, CA
125), enzymes (amylase/lipase) and DNA quality/content or mutational
analysis. Mutational analysis can further characterize allelic
imbalances, loss of heterozygosity (LOH) and K-ras mutation. Estimates
of the cyst fluid volume can be approximated using the formula 4r3;
where r is the radius of the cyst. Imaging is the less accurate in the
diagnosis of pancreatic cystic lesions. Surgical resection is
recommended if a cyst has both a solid component and dilated main
pancreatic duct. Viscosity is lower in pseudocysts (1.3) and serous
cystadenomas (1.27), compared with mucinous cystadenoma (1.84) and
mucinous cystadenocarcinomas (1.90). CEA levels are high in mucinous
cystadenomas and very high in mucinous cystadenocarcinomas when
compared with pseudocysts and serous cystadenomas. Elevated CEA and
viscosity accurately predict mucinous cysts. The presence of
extracellular mucin is predictive of a mucinous neoplasm. Cytological
identification of extracellular mucin and CEA elevation are predictors
of mucinous neoplasm and malignancy. Cytology detects mucin containing
cells, malignant cells, glycogen-rich cuboidal cells, branching
papillae and abundant enucleate squamous cells and debris. Cytology is
diagnostic in less than 60% of pancreatic cysts. CA19-9 cyst fluid
levels above 50,000 U/ml are found in mucinous cystadenoma and
cystadenocarcinomas. Levels below 37 U/ml suggest serous cystadenomas
or pseudocysts. CA 19-9 is suitable for detection of malignancy but is
insensitive for premalignant lesions. CA72-4 cyst fluid levels above 40
U/ml are significantly high in mucinous cystic tumors. CEA levels
greater than 400 ng/ml are characteristic of mucinous tumors and
cystadenocarcinoma, while CEA levels less than 4 ng/ml are associated
with serous cystadenomas. Cyst fluid CEA is the most accurate test
available for diagnosis of mucinous cystic lesions of the pancreas with
a cut off value of 192 ng/ml for diagnosis. In some centers CEA is the
only tumor marker routinely use for diagnostic work-up. Amylase/lipase
high levels (above 250 U/L) are commonly seen in pseudocysts. Cyst
fluid amylase is of limited utility in evaluation of pancreatic cysts.
Low values are associated with a neoplastic tumor. DNA analysis has
found that a K-ras mutation followed by allelic loss is most predictive
of malignancy in a pancreatic cyst. In malignant cysts, elevated CEA is
more predictive of histology than K-ras or LOH mutations. DNA
mutational analysis should be used selectively rather than routinely.
Costs are significant with DNA analysis. Confocal laser endomicroscopy
(CLE) is a novel technology for real time in vivo microscopic imaging
using a probe inserted through a 19-G needle. CLE findings of mucinous
tumors include finger-like papillae with layers of a thick band-like
epithelium. Serous cysts have a superficial network or fern-type
pattern, while pseudocysts contain bright particles. Cytology, CEA
levels and -K-ras mutation analyses are the most important in clinical
practice. Is important differentiate between benign and malignant
lesions to determine whether surgical resection or conservative
management is required. (See attached Table).
 References:
References:
1- Bhutani MS, Gupta V, Guha S, Gheonea DI, Saftoiu A.
Pancreatic cyst fluid analysis--a review. J Gastrointestin Liver Dis.
20(2):175-80, 2011
2- Rockacy M, Khalid A. Update on pancreatic cyst fluid analysis. Ann Gastroenterol. 26(2):122-127, 2013
3- Talar-Wojnarowska R, Pazurek M, Durko L, et al: Pancreatic cyst
fluid analysis for differential diagnosis between benign and malignant
lesions. Oncol Lett. 5(2):613-616, 2013
4- Ngamruengphong S, Lennon AM. Analysis of Pancreatic Cyst Fluid. Surg Pathol Clin. 9(4):677-684, 2016
5- Li F, Malli A, Cruz-Monserrate Z, Conwell DL, Krishna SG. Confocal
endomicroscopy and cyst fluid molecular analysis: Comprehensive
evaluation of pancreatic cysts. World J Gastrointest Endosc. 10(1):1-9,
2018
6- Arner DM, Corning BE, Ahmed AM, et al: Molecular analysis of
pancreatic cyst fluid changes clinical management. Endosc Ultrasound.
7(1):29-33, 2018
PSU Volume 56 NO 03 MARCH 2021
Complicated Appendicitis: How much antibiotics?
Appendicitis is the most common acute surgical emergency in
children. Though medical management of appendicitis using parenteral
antibiotics has gained popularity, laparoscopic or open appendectomy is
still the standard of care. The basic tents of management of
appendicitis are: resuscitate children with systemic inflammatory
response syndrome, control the source of contamination, remove most of
the infected or necrotic material and administer antimicrobial agents
to eradicate residual pathogens. Appendicitis can be classified as
simple or complicated. Complicated appendicitis refers to histologic
changes in the appendix associated with either gangrene and/or
perforation. Perforated appendicitis occurs in approximately 25-30% of
children presenting with acute appendicitis. Traditionally, complicated
appendicitis is managed postoperatively with 7-14 days of antibiotics.
This long therapeutic regimen has been challenged periodically. The
main reasons for postoperative antibiotics after appendectomy in
complicated appendicitis is reducing the incidence of surgical
site (wound) infection and formation of intraabdominal fluid
collections (abscess). Excluding gangrenous appendicitis, wound and
intraabdominal infection rates for children with perforated
appendicitis are 4% and 8% respectively. With the standard use of
laparoscopy with smaller incisions for appendectomy the surgical site
infection rate has decreased considerably. All children undergoing
appendectomy should receive a preoperative dose of a broad-spectrum
antibiotics before surgery. Cases with simple appendicitis or normal
appendix do not need to receive postoperative antibiotics as
postoperative infectious complications are extremely rare in this group
after a single preoperative dose of antibiotics. Complicated
appendicitis can be managed with shorter course of three to 5 days of
postoperative antibiotics after adequate source control. This course
depends on the clinical response of the child. In synthesis, the
parameters utilized to evaluate the clinical response to postoperative
antibiotic therapy include: temperature, heart rate, WBC count, and
gastrointestinal dysfunction due to peritonitis. This means that until
fever subsides (T < 38.5 C), normal heart rate returns, WBC < 11
and resolution of ileus with oral intake is not achieved, the child
will need antimicrobial therapy. The STOP-IT trial found that outcomes
in patients with intraabdominal infections who undergo successful
source control procedure and received a fixed 4 days course of
antimicrobial therapy had similar results in outcome as patient whom
systemic antimicrobial agents were administered until after resolution
of signs and symptoms of sepsis. The occurrence of a postoperative
abscess is the single most important determinant of outcomes in
children with perforated appendicitis. Retained fecalith after
appendectomy are a source of continued and recurrent infection and
should be removed surgically. As we define better grades of perforation
during surgery, we will be able to correlate them with increased
postoperative abscess rate.
References:
1- Neilson IR, Laberge JM, Nguyen LT, et al: Appendicitis in Children:
Current Therapeutic Recommendations. J Pediatr Surg. 25(11): 1113-1116,
1990
2- Emil S, Laberge JM, Mikhail P, et al: Appendicitis in Children: A
ten-Year Update of Therapeutic Recommendations. J Pediatr Surg. 38(2):
236-242, 2003
3- Emil S, Taylor M, Ndiforchu F, Nguyen N: What are the True
Advantages of a Pediatric Appendicitis Clinical Pathway? Am Surg. 72
(10): 885-889, 2006
4- Sawyer RG, Claridge JA, Nathens AB, et al: Trial of Short-Course
Antimicrobial Therapy for Intraabdominal Infection. NEJM 372:
1996-2005, 2015
5- Emil S, Elkady S, Shbat L, et al: Determinants of postoperative
abscess occurrence and percutaneous drainage in children with
perforated appendicitis. Pediatr Surg Int. 30: 1265-71, 2014
6- Yousef Y, Youssef F, Homsy M, et al: Standardization of care for
pediatric perforated appendicitis improves outcomes. J Pediatr Surg 52:
1916-1920, 2017
7- Posillico SE, Young BT, Ladhani HA, Zosa BM, Claridge JA:
CurrentEvaluation of Antibiotic Usage in Complicated Intra-Abdominal
Infection after the STOP IT Trial: Did We STOP IT?. Surg Infect 20(3):
184-191, 2019
Intraoperative Cholangiogram
Cholelithiasis has increased in incidence in the pediatric
population, mostly the result of western diet and improve ability to
detect them using ultrasonography. Cholelithiasis can cause biliary
colicky pain, acute and chronic inflammation, pancreatitis and biliary
obstruction from gallstone impaction in the common bile duct
(choledocholithiasis). Hemolytic disorders account for 20% of children
with cholelithiasis. Laparoscopic cholecystectomy (LC) is the gold
standard method for gallbladder removal in children and adults with
disease gallbladder. In children with gallstone pancreatitis,
biochemical jaundice, cholangitis or ultrasound evidence of bile duct
dilatation an MRCP should be performed to diagnosed choledocholithiasis
before embarking in removal of the gallbladder. Laparoscopic removal of
the gallbladder with bile duct obstruction could result in leakage of
the cystic duct stump or development of cholangitis. In the
laparoscopic era, ERCP with sphincterotomy is used either before LC for
suspected common bile duct (CBD) stones or after LC for missed CBD
stones, bile duct injuries or late strictures. Intraoperative
cholangiogram (IOC) is a technique in which a small catheter is passed
either through the cystic duct or infundibulum of the gallbladder,
contrast is injected and fluoroscopic films of the biliary tree are
obtained. IOC was first reported in the early 1990's. Previous
indications for IOC include findings of bile duct dilatation, defining
aberrant biliary tree anatomy, in cases of confused anatomy,
confounding biliary disorders (choledochal cysts, biliary atresia or
other congenital anomalies), and when bile duct injury is suspected
during cholecystectomy. In places where MRCP is not available, IOC is
useful to identify biliary anomalies or obstruction causing
choledocholithiasis, cholangitis, elevated liver enzymes or
pancreatitis. With time, IOC has gone from a routine procedure to a
selective procedure during removal of the gallbladder in children. IOC
adds time to the procedure potentially increasing costs, puts the
patient at risk of iatrogenic injury, adds radiation exposure to the
patient, and is no more sensitive than preoperative MRCP. Recent
nationwide database studies of cholecyctectomies in children have found
that:1) Routine IOC is more commonly employed in children with
cholecystitis and less commonly performed in patents with
cholelithiasis. 2) Cholecystectomy alone when compared against
cholecystectomy with routine IOC is associated with higher rates of
bile duct injury, perforation, laceration, sepsis and other infections.
3) These children are less likely to have readmission to the hospital
within 30 days and one year. 4) Patients who undergo routine IOC have
decreased hospital length of stay and decreased overall index hospital
costs. Other studies have found IOC and hospital operative volume are
not readily associated with decreased bile duct injury. Increase use of
IOC or tendency is associated with increased risk for bile duct injury.
The use of IOC is associated with surgeons' preference and training as
there is no clear evidence to guide routine
utilization.
References:
1- Lugo-Vicente HL: Trends in Management of Gallbladder Disorders in Children. Pediatr Surg Int. 12(5-6): 348-352, 1998
2- Waldhausen JH, Graham DD, Tapper D: Routine intraoperative
cholangiography during laparoscopic cholecystectomy minimizes
unnecessary endoscopic retrograde cholangiopancreatography in children.
J Pediatr Surg. 36(6):881-4, 2001
3- Mah D, Wales P, Njere I, et al: Management of suspected common bile
duct stones in children: role of selective intraoperative cholangiogram
and endoscopic retrograde cholangiopancreatography. J Pediatr Surg.
39(6):808-12, 2004
4- Hamad MA, Nada AA, Abdel-Atty MY, Kawashti AS: Major biliary
complications in 2,714 cases of laparoscopic cholecystectomy without
intraoperative cholangiography: a multicenter retrospective study. Surg
Endosc. 25(12):3747-51, 2011
5- Kelley-Quon LI, Dokey A, Jen HC, Shew SB: Complications of pediatric
cholecystectomy: impact from hospital experience and use of
cholangiography. J Am Coll Surg. 218(1):73-81, 2014
6- Martin B, Ong EGP: Selective intraoperative cholangiography during
laparoscopic cholecystectomy in children is justified. J Pediatr Surg.
53(2):270-273, 2018
7- Quiroz HJ, Valencia SF, Willobee BA, et al: Utility of routine
intraoperative cholangiogram during cholecystectomy in children: A
nationwide analysis of outcomes and readmissions. J Pediatr Surg.
56(1):61-65, 2021
Appendicitis during Covid19 Pandemic
Acute appendicitis is the most common surgical emergency in
children. More than 70,000 appendectomies are performed in children
each year in the USA, approximately one-third of childhood admissions
for abdominal pain. Since the discovery of Sars-Cov2 coronavirus as the
cause of Covid19 disease early in 2020, a global pandemic developed
which continues to affect medical and surgical care of children and
adults. Children who develop Covid19 mostly suffer from a mild disease
process, only a minority presenting with respiratory distress syndrome
or multiorgan failure. With the pandemic the instructions to the
population were to stay home, avoid visiting local clinics and
hospitals while using more telemedicine-based practice. In many
institutions programmed or elective surgery was postponed. Parental
concerned with the possibility of contracting Covid19 in public places
such as clinics or emergency rooms couple with inadequate clinical
evaluation using telemedicine, and the inability to perform a full
physical examination led to a delayed diagnosis of appendicitis. This
resulted in an increase in development of complex appendicitis
(gangrenous and perforated) along with an increase in intraabdominal
complications such as perforation and peritonitis with
peri-appendicular abscess formation. An increased reliance on
outpatient care during a time when many clinics were not seeing
patients most likely contributed to delay in diagnosis. As hospital
systems were reaching capacity, a delay in presentation was created due
to the need for transfer from one health care center to another. A few
institutions changed to non-operative management of appendicitis in an
effort to conserve resources, minimized non-emergent surgical
procedures and allow for Covid19 testing to result. Non-operative
management with intravenous antibiotics can be applied to almost 50% of
children presenting with acute appendicitis. Children with persistent
pain, leukocytosis or an appendicolith in images were taken directly to
the operating room for laparoscopic appendectomy. The observed increase
in complicated appendicitis cases in children during 2020 is most
likely due to Covid19 pandemic. In other institutions the covid19
pandemic increased the number of children managed for acute
appendicitis during the lock down due to closure of general surgery
departments in proximity hospital resulting in an increase in referral
to children hospitals. Children managed for acute appendicitis were
older than historic controls and more commonly transferred from other
institutions. Nonoperative management of uncomplicated appendicitis in
children resulted in an increase in length of stay and readmission.
Ages at presentation correlated with increased severity of disease on
presentation and higher rates of perforation and intraabdominal abscess
formation, increased use of antibiotics, longer length of stay and
longer duration until symptoms resolution for children managed during
the pandemic. Children with appendicitis presented during the pandemic
with more advanced disease.
References:
1- Snapiri O, Rosenberg Danziger C, Krause I, et al: Delayed diagnosis
of paediatric appendicitis during the COVID-19 pandemic. Acta Paediatr.
109(8):1672-1676, 2020
2- Kvasnovsky CL, Shi Y, Rich BS, et al: Limiting hospital resources
for acute appendicitis in children: Lessons learned from the U.S.
epicenter of the COVID-19 pandemic. J Pediatr Surg. 2020 Jun
23:S0022-3468(20)30444-9. doi:10.1016/j.jpedsurg.2020.06.024.
3- Montalva L Haffreingue A, Ali L, et al: The role of a pediatric
tertiary care center in avoiding collateral damage for children with
acute appendicitis during the COVID-19 outbreak. Pediatr Surg Int.
36(12):1397-1405, 2020
4- Gerall CD, DeFazio JR, Kahan AM, et al: Delayed presentation and
sub-optimal outcomes of pediatric patients with acute appendicitis
during the COVID-19 pandemic. J Pediatr Surg. 2020 Oct
19:S0022-3468(20)30756-9. doi:10.1016/j.jpedsurg.2020.10.008.
5- La Pergola E, Sgro A, Rebosio F, et al:Appendicitis in Children in a
Large Italian COVID-19 Pandemic Area. Front Pediatr. 2020 Dec
9;8:600320. doi: 10.3389/fped.2020.600320.
6- Place R, Lee J, Howell J: Rate of Pediatric Appendiceal Perforation
at a Children's Hospital During the COVID-19 Pandemic Compared With the
Previous Year. JAMA Netw Open. 2020 Dec 1;3(12):e2027948.
doi:10.1001/jamanetworkopen.2020.27948.
7- Tankel J, Keinan A, Blich O, et al: The Decreasing Incidence of
Acute Appendicitis During COVID-19: A Retrospective Multi-centre Study.
World J Surg. 44(8):2458-2463, 2020
PSU Volume 56 NO 04 APRIL 2021
Abdominal Wall Defects and Undescended Testis
The most common congenital abdominal wall defects (AWD) in
children affecting testicular descent include gastroschisis,
omphalocele and prune belly syndrome. Gastroschisis is more
common than omphalocele or prune belly syndrome. Undescended testes are
more common among AWD patients than the general population, with the
highest prevalence in omphalocele. Testicular descent is known to be
related to intraabdominal pressure and the gubernaculum. Without higher
pressures the forces that encourage testicle migration are absent.
Failure of appropriated abdominal pressure and/or sudden disruption of
the gubernaculum at the time of formation can lead to abdominal
undescended testes. Undescended testes are a concomitant birth anomaly
associated with abdominal wall defects in almost 20 to 40% of all males
patients. Undescended testes in children with abdominal wall defects
may be found within or outside the abdominal cavity. The majority of
undescended testes are found at the internal spermatic ring.
Gestational age and birth weight do not appear to be a significant
factor associated with testicular maldescent in several series. Closure
of the abdominal wall defect using either mesh or primary closure is
initially warranted. After closure of the abdominal wall defect and in
the ensuing next 12 months, more than half of all undescended testes
migrate to the appropriate position at follow-up requiring no
intervention. Migration of the testes into the scrotum in cases of
left-sided undescended testes born with gastroschisis, along with most
in omphalocele is less likely. Of the testes that do not spontaneously
descend into the scrotum, nearly half (43%) migrated into the inguinal
canal. In cases where the testes remained in the abdominal cavity
(non-palpable), laparoscopy is successfully performed to localize and
remove or reposition the testes. Early conservative management allows
normal spontaneous descent in most testes. Testes that are
extraabdominal at birth appear to be less likely to spontaneously
migrate into the scrotum compared with those that are intraabdominal at
birth. Extraabdominal testes seem to have a greater incidence of
atrophy and need for orchiectomy. Babies with prolapse testes out of
the abdominal cavity should undergo manual repositioning placing the
testis as near as possible to the internal spermatic ring. It is
important to record the position of the intraabdominal undescended
testes at the time of abdominal wall repair because future diagnostic
laparoscopy or exploration is likely to be difficult. The majority will
descend on their own without any need for surgical intervention.
Orchiopexy in a newborn with fragile testicular vessels and the risk of
compromised blood flow in the presence of temporarily raised
intracoelomic pressure after primary closure may jeopardize testicular
viability. Other authors believe that early mobilization and fixation
can improve the outcome of the undescended testis. Surgical
intervention for an undescended testis after closure of AWD is
challenging, characterized by adhesions and short gonadal vessels
necessitating staging the descent.
References:
1- Lawson A, de La Hunt MN: Gastroschisis and undescended testis. J Pediatr Surg. 36(2):366-7, 2001
2- Chowdhary SK, Lander AD, Buick RG, Corkery JJ, Gornall P: The
primary management of testicular maldescent in gastroschisis. Pediatr
Surg Int. 17(5-6):359-60, 2001
3- Hill SJ, Durham MM: Management of cryptorchidism and gastroschisis. J Pediatr Surg. 46(9):1798-803, 2011
4- Yardley IE, Bostock E, Jones MO, Turnock RR, Corbett HJ, Losty PD:
Congenital abdominal wall defects and testicular maldescent--a 10-year
single-center experience. J Pediatr Surg. 47(6):1118-22, 2012
5- Raitio A, Syvanen J, Tauriainen A, et al: Congenital abdominal wall
defects and cryptorchidism: a population-based study. Pediatr Surg Int.
2021 Jan 31. doi: 10.1007/s00383-021-04863-9.
6- Berger AP, Hager J: Management of neonates with large abdominal wall
defects and undescended testis. Urology. 68(1):175-8, 2006
Congenital Anal Stenosis
Congenital anal stenosis is a rare disorder classified as a
low anorectal malformation, mainly a short stenosis in most cases, but
sometimes the child has a funnel shape long stenosis associated with a
presacral mass, bony sacral defect, anterior meningocele or other
anomaly. The type of sacral dysplasia is scimitar and the presacral
tumor is a mature teratoma in most of the cases. Children with
congenital anal stenosis suffer from chronic intractable constipation,
soiling, encopresis and megarectosigmoid. Constipation is the most
common early functional problem in children with anal stenosis in more
than 40% of the cases. Recalcitrant constipation is more common in
children with a delayed diagnosis and treatment of anal stenosis.
Congenital anal stenosis (CAS) can also be associated with
Mayer-Rokitansky-Kuster-Hauser syndrome. On physical examination,
children with CAS have a stenotic opening at the normal anal site
barely admitting a small Hegar dilator. Palpable fecalomas can be found
upon abdominal examination. In this malformation, the anal canal is
usually located at least partially inside the voluntary sphincter
funnel. Occasionally the diagnosis of anal stenosis is delayed to later
infancy, especially in cases where the bowel outlet is stenotic but at
or near the proper anal position. Newborns with anal stenosis usually
pass meconium in the first 48 hours after birth. Children born with CAS
should undergo an active search for associated malformations including
echocardiogram, ultrasound of the spinal cord and kidneys,
cystourethrogram and imaging of the entire spine including the sacrum
(MRI). CAS can be managed with gradual Hegar dilatations usually
without the need for anesthesia. Serial dilatations are started with a
Hegar dilator that is easily fitted into the anal opening, usually with
a size between six and 8 mm. Dilatations are taught to parents to
continue management increasing to the next number on a weekly basis,
continued for six weeks after the age-appropriate size of the anus is
reached. Currarino syndrome, dysganglionosis including Hirschsprung's
disease and chromosomal defects commonly occurs in children with funnel
anus. The mortality of patients with low anomaly as CAS, is about three
times lower than that of patients with high anomalies, and usually
associated to cardiac defects. Long-term results of low malformations
are usually good in most patients. Poor results are usually associated
to neurological damage, mental retardation or insufficient care of
patients. In severe cases of anal stenosis, the posterior rectum is
mobilized in the form of rectal advancement, and the posterior 180
degrees is anastomosed directly to the skin with preservation of the
anal canal as the anterior final anoplasty. These patients have an
excellent prognosis for bowel control and fecal continence, and
therefore, complete mobilization and resection of the anal canal must
be avoided. Those children with CAS and stenosis involving only the
skin-level can be managed with a Heineke-Mikulicz anoplasty with very
good results.
References:
1- Suomalainen A, Wester T, Koivusalo A, Rintala RJ, Pakarinen MP:
Congenital funnel anus in children: associated anomalies, surgical
management and outcome. Pediatr Surg Int. 23(12):1167-70, 2007
2- Joshi M, Singh S, Vyas T, Chourishi V, Jain A:
Mayer-Rokitansky-Kuster-Hauser syndrome and anal canal stenosis: case
report and review of literature. J Pediatr Surg. 45(12):e29-31, 2010
3- Pakarinen MP, Rintala RJ: Management and outcome of low anorectal malformations. Pediatr Surg Int. 26(11):1057-63, 2010
4- Lane VA Wood RJ, Reck C, Skerritt C, Levitt MA: Rectal atresia and
anal stenosis: the difference in the operative technique for these two
distinct congenital anorectal malformations. Tech Coloproctol.
20(4):249-54, 2016
5- Halleran DR, Sanchez AV, Rentea RM, et al: Assessment of the
Heineke-Mikulicz anoplasty for skin level postoperative anal strictures
and congenital anal stenosis. J Pediatr Surg. 54(1):118-122, 2019
6- Brem H, Beaver BL, Colombani PM, et al: Neonatal diagnosis of a
presacral mass in the presence of congenital anal stenosis and partial
sacral agenesis. J Pediatr Surg. 24(10):1076-8, 1989
Appendix Duplication
The vermiform appendix is a tubular, narrow, worm-shaped
part of the alimentary canal that lies near the ileocecal junction and
communicates with the cecum. The appendix develops as a conical
extension from the apex of the cecal diverticulum which arises from the
antimesenteric border of the proximal part of the post arterial segment
of the midgut. Anomalies associated with the appendix are rare.
Duplication of the vermiform appendix is very rare occurring with an
incidence of 0.0004% after appendectomy. Appendiceal duplication is
classified into three types (Cave-Wallbridge classification). Type A
consists of various degrees of partial duplication on a normally
localized appendix with a single cecum. Type B consists of a single
cecum with two completely separate appendixes. This type is further
subdivided into a B1, bird-like type if the two appendixes are located
symmetrical on either side of the cecum as usually occurs in birds, and
B2 also known as tenia-coli type which has a normally located appendix
arising from the cecum at the usual site and a second separate
rudimentary appendix located along the line of one of the tenias. B1 or
bird-like type of duplication is the most common type of appendiceal
duplication (37%). Type B2 duplication can be mimicked by a solitary
inflamed diverticulum found on the cecum, usually at the medial border
just above the ileocecal junction. B3 if the second appendix is located
along the tenia of the hepatic flexure of the colon, and B4 if the
location of the second appendix is along the tenia of the splenic
flexure of the colon. Type C consists of a duplicated cecum, each with
an appendix. A horseshoe configuration of duplication and triple
appendices have also been described. Appendiceal duplication is most
commonly identified incidentally during surgery or at autopsies. The
most common presentation is appendicitis with the duplication being
discovered intraoperatively. Median age at presentation of these
patients is adolescent years, males and females affected equally.
CT-Scan is the best mode of imaging to identify a duplicated
appendix. Besides inflammation, appendiceal duplication can
present with recurrent intussusception or an appendiceal mass. Surgeons
have to be aware of such anomalies since a second laparotomy revealing
a previously removed appendix can cause medicolegal situations. In
cases with appendiceal duplication, when only one appendix is inflamed,
both should be removed to avoid a diagnostic dilemma that may arise
later.
References:
1- Varshney M, Shahid M, Maheshwari V, Mubeen A, Gaur K: Duplication of
appendix: an accidental finding. BMJ Case Rep. 2011 Mar
8;2011:bcr0120113679. doi: 10.1136/bcr.01.2011.3679.
2- Marshall AP, Issar NM, Blakely ML: Aapendiceal duplication in
children presenting as a appendiceal tumor and as recurrent
intussusception. J Pediatr Surg. 48: E9-E12, 2013
3- Dubhashi SP, Dubhashi UP, Kumar H, Patil C: Double Appendix. Indian J Surg. 77(Suppl 3):1389-90, 2015
4- Bhat GA, Reshi TA, Rashid A: Duplication of Vermiform Appendix. Indian J Surg. 78(1):63-4, 2016
5- Nageswaran H, Khan U, Hill F, Maw A: Appendiceal Duplication: A
Comprehensive Review of Published Cases and Clinical Recommendations.
World J Surg. 42(2):574-581, 2018
6- Chew DK, Borromeo JR, Gabriel YA, Holgersen LO: Duplication of the vermiform appendix. J Pediatr Surg. 35(4):617-8, 2000
PSU Volume 56 No 05 MAY 2021
Eosinophilic Cholecystitis
Eosinophilic cholecystitis is described as acute acalculous
cholecystitis associated with infiltrations of eosinophils into the
gallbladder wall. Acalculous cholecystitis is a rare condition in
children. It occurs during the course of infectious disease, as well as
in children on total parenteral nutrition, after surgery, trauma and
extensive burns. The etiology of acalculous cholecystitis includes
decreased blood flow to the gallbladder, biliary tract obstruction and
hyperconcentration of the bile. The disease can progress to necrosis or
perforation of the gallbladder. Eosinophilic cholecystitis (EC) is very
rare, reported in 0.5-6.5% of removed gallbladders. The etiology of
eosinophilic cholecystitis is unknown and probably represents a
hypersensitivity type of inflammatory response to altered bile.
Histologic findings diagnostic of eosinophilic cholecystitis includes
transmural infiltration of leukocytes with more than 90% eosinophils
present. Known causes of eosinophilic cholecystitis include parasitic
infestation (clonorchis sinensis, echinococcus and Ascaris
lumbricoides), gallstones, allergies, reaction to certain medications
(erythromycin, L-tryptophan and cephalosporins), allergic granulomatous
vasculitis, and association with eosinophilic gastroenteritis and/or
eosinophilic pancreatitis. Gallstones can be found in almost 90% of the
eosinophilic cholecystitis cases. Eosinophilic cholecystitis is more
common in adult females between the ages of 25-64 years. In children,
most cases occur during teenage years, though patient as young as seven
years of age has been reported. Symptoms of eosinophilic cholecystitis
resembles those of acute cholecystitis, including pain in the upper
right quadrant, fever, jaundice, Murphy positive sign, altered mental
status, shock, leukocytosis and postprandial nausea and vomiting.
Eosinophilic cholecystitis can be diagnosed only on resection of the
gallbladder and histologic examination. The diagnosis can be suspected
if there is evidence of peripheral eosinophilia which occurs in 10-15%
of cases. Ultrasound of the abdomen shows features of calculus
cholecystitis with pericholecystic fluid collection and edema,
thickened gallbladder wall and dilated common bile duct. The treatment
of choice of eosinophilic cholecystitis is removal of the sick
gallbladder. Cases associated with eosinophilic gastroenteritis or
cholangitis, can benefit from steroids medication as adjuvant therapy.
Eosinophilic cholecystitis usually show a good prognosis and patients
with cholecystitis alone improve after cholecystectomy.
References:
1- Muta Y, Odaka A, Inoue S, Komagome M, Beck Y, Tamura M, Arai E:
Acute acalculous cholecystitis with eosinophilic infiltration. Pediatr
Int. 57(4):788-91, 2015
2- Khan S, Hassan MJ, Jairajpuri ZS, Jetley S, Husain M.
Clinicopathological Study of Eosinophilic Cholecystitis: Five Year
Single Institution Experience. J Clin Diagn Res. 11(8):EC20-EC23, 2017
3- Gutierrez-Moreno LI, Trejo-Avila ME, Diaz-Flores A, et al:
Eosinophilic cholecystitis: a retrospective study spanning a
fourteen-year period. Rev Gastroenterol Mex. 83(4):405-409, 2018
4- Keyal NK, Adhikari P, Baskota BD, Rai U, Thakur A: Eosinophilic
Cholecystitis presenting with Common Bile Duct Sludge and Cholangitis:
A Case Report.J Nepal Med Assoc 58(223): 188-91, 2020
5- Ito H, Mishima Y, Cho T, et al: Eosinophilic Cholecystitis
Associated with Eosinophilic Granulomatosis with Polyangiitis. Case Rep
Gastroenterol. 14(3):668-674, 2020
6- Garzon G LN, Jaramillo B LE, Valero H JJ, Quintero C EM:
Eosinophilic cholecystitis in children: Case series. J Pediatr
Surg. 56(3):550-552, 2021
Ovarian Harvest
With the advent of newer therapeutic options and increase in
intensity of such therapy more than 80% of children diagnosed with
cancer will be long-term survivors. During management for cancer
children will suffer long-term adverse effects such as loss of
fertility. Treatment protocols highly deleterious for ovarian function
include high dose alkylating agents (cyclophosphamide and busulfan),
total body irradiation and abdominopelvic irradiation (5-20 Greys) that
includes both ovaries. Ovarian damage is drug- and dose-dependent and
increases with age at treatment. For prepubertal females, ovarian
harvest using tissue cryopreservation is currently the only available
means of potentially preserving gonad function and fertility. Ovarian
tissue harvesting can take place immediately before intensive
chemotherapy or irradiation. Ovarian tissue is collected during the
removal of a primary abdominal tumor, or by means of a small suprapubic
laparotomy or laparoscopy. A single ovary or 2/3 of each ovary is
removed for cryopreservation. Most cases of major bleeding requiring
transfusion or re-operation are associated with partial oophorectomy.
Since the ovary can be different in the number of follicles present,
some people prefer specimen collection from both ovaries. During the
surgical procedure it is also possible to move residual gonads in order
to reduce effects of local therapy (ovarian transposition). The
preserved ovary is frozen down to liquid nitrogen temperature. Using a
biopsy of the cortex of the removed ovary the number of primordial and
primary follicles per square mm is determined. Also, in all malignant
cases small samples of gonadal tissue are also sent for routine
pathological assessment to rule out any gonadal involvement by the
primary cancer. Ovarian tissue is preserved until the child recovers
and it is reimplanted. Primordial follicles can be isolated from
cryopreserved ovarian tissue and grown to maturity in vitro to be
utilized for in vitro fertilization and embryo transfer. Orthotropic
sites of reimplantation are the residual ovary or a peritoneal pocket
in the ovarian fossa that permit a spontaneous pregnancy which is not
recommended in case of pelvic irradiation. Heterotrophic sites are the
subcutaneous forearm or abdominal tissue. Ovarian tissue transplant,
whether orthotopic or heterotopic, would allow for ovarian hormonal
production and restoration of a normal hormonal milieu allowing future
pregnancy. Orthotropic ovarian reimplantation has led to the birth of
more than 130 healthy babies. Mature oocyte and embryo cryopreservation
is an appropriate strategy for fertility preservation in postpubertal
females. Oocytes can be collected by transvaginal oocyte pick up, from
excised ovarian tissue or a combination of both procedures. However
this approach is time consuming with a low pregnancy rate and does not
replace ovarian tissue transplantation.
References:
1- Poirot CJ, Martelli H, Genestie C, et al: Feasibility of ovarian
tissue cryopreservation for prepubertal females with cancer. Pediatr
Blood Cancer. 49(1):74-8, 2007
2- Detti L, Martin DC, Williams LJ: Applicability of adult techniques
for ovarian preservation to childhood cancer patients. J Assist
Reprod Genet. 29(9):985-95, 2012
3- Babayev SN, Arslan E, Kogan S, Moy F, Oktay K: Evaluation of ovarian
and testicular tissue cryopreservation in children undergoing
gonadotoxic therapies. J Assist Reprod Genet. 30(1):3-9, 2013
4- Lima M, Gargano T, Fabbri R, Maffi M, Destro F: Ovarian tissue
collection for cryopreservation in pediatric age: laparoscopic
technical tips. J Pediatr Adolesc Gynecol. 27(2):95-7. 2014
5- Kedem A, Yerushalmi GM, Brengauz M, et al: Outcome of immature
oocytes collection of 119 cancer patients during ovarian tissue
harvesting for fertility preservation. J Assisted Reprod and Genetics.
35:851-856, 2018
6- Corkum KS, Rhee DS, Wafford QE, et al: Fertility and hormone
preservation and restoration for female children and adolescents
receiving gonadotoxic cancer treatments: A systematic review. J Pediatr
Surg. 54(11):2200-2209, 2019
7- Lukish JR: Laparoscopic assisted extracorporeal ovarian harvest: A
novel technique to optimize ovarian tissue for cryopreservation in
young females with cancer. J Pediatr Surg. 56(3):626-628, 2021
Minimally Invasive Follicular Thyroid Carcinoma
Papillary and follicular thyroid carcinomas are considered
the two differentiated carcinomas arising from follicular cells most
commonly found in children. Papillary thyroid carcinoma accounts for
more than 95% of all cases. Follicular thyroid carcinoma (FTC) accounts
for less than 5% of all cases, occur more commonly in females, and is
characterized by capsular and vascular invasion precluding making a
categorical diagnosis using fine needle aspiration (FNA) biopsy. FNA
biopsy usually describes a follicular tumor and the child undergoes
removal of the affected lobe with the tumor. FTC is known to be
associated with TSH elevation, iodine deficiency areas, hemiagenesis
and radiation exposure. Tumor size of pediatric FTC is significant
larger than in adult FTC. Histology of pediatric FTC is classified into
five patterns: microfollicular, follicular, solid/trabecular, oncocytic
and mixed patterns. Microfollicular pattern is the most common, while
solid/trabecular the most rare. In FTC, RAS mutation is the most common
genetic alteration with a prevalence in children of 12%. RAS mutation
is associated with smaller tumor size with a potential low risk
behavior in children. Follicular thyroid carcinoma can be further
divided into minimally invasive and widely invasive. Minimally invasive
FTC is diagnosed histologically when microscopic penetration of the
tumor capsule is found but there is no vascular invasion. Minimally
invasive FTC is difficult to diagnose prior to thyroidectomy unless
distant metastases to bone or lung and/or lymph nodes have been
detected and diagnosed by FNA or further imaging. Definitive diagnosis
is established after hemithyroidectomy. Minimally invasive FTC is an
encapsulated neoplasm characterized by unequivocal capsular invasion
with a relatively uneventful and indolent course. Minimally invasive
FTC carries an excellent prognosis with low risk of recurrence or
disease-specific mortality. Removal of the affected lobe and five-year
ultrasound-guided observation is sufficient therapy. Widely invasive
FTC is more aggressive, shows a widespread infiltration of blood
vessels into the adjacent thyroid parenchyma, displaying a poorer
prognosis than minimally invasive variant. Completion total
thyroidectomy as a second surgery and radioactive iodine ablation is
recommended for invasive FTC. Poor prognostic factors associated with
follicular thyroid carcinoma include older age, distant and neck
metastasis. Children with tumor exceeding 4 cm in length and associated
with vascular invasion carries a poorer
prognosis.
References:
1- Sugino K, Kameyama K, Ito K, et al: Outcomes and prognostic factors
of 251 patients with minimally invasive follicular thyroid carcinoma.
Thyroid. 22(8):798-804, 2012
2- Ito Y, Miyauchi A, Tomoda C, Hirokawa M, Kobayashi K, Miya A:
Prognostic significance of patient age in minimally and widely invasive
follicular thyroid carcinoma: investigation of three age groups. Endocr
J. 61(3):265-71, 2014
3- Stenson G, Nilsson IL, Mu N, et al: Minimally invasive follicular
thyroid carcinomas: prognostic factors. Endocrine. 53(2):505-11, 2016
4- Zou CC(1), Zhao ZY, Liang L: Childhood minimally invasive follicular
carcinoma: clinical features and immunohistochemistry analysis. J
Paediatr Child Health. 46(4):166-70, 2010
5- Vuong HG, Kondo T, Oishi N, et al: Paediatric follicular thyroid
carcinoma - indolent cancer with low prevalence of RAS mutations and
absence of PAX8-PPARG fusion in a Japanese population. Histopathology.
71(5):760-768, 2017
6- Nicolson NG, Murtha TD, Dong W, et al: Comprehensive Genetic
Analysis of Follicular Thyroid Carcinoma Predicts Prognosis Independent
of Histology. J Clin Endocrinol Metab. 103(7):2640-2650, 2018
PSU Volume 56 NO 06 JUNE 2021
Neurothekeoma
Soft tissue tumor lesions are uncommon in the pediatric age
group. They differ from adults counterpart in frequency, anatomical
site and prognosis. In children, a dermal nodule could represent a
fibrous tumor, histiocytic tumor, lymphocytic tumor, melanocytic tumor
or a tumor of neural origin. Neurothekeoma is a rare benign soft tissue
tumor with distinctive histological features commonly located on the
upper extremity or the head and neck region in children. Originally
thought to arise from peripheral nerve sheath, it has been postulated
that neurothekeomas are of fibrohistiocytic differentiation. Based on
histology, immunohistochemical findings and amount of myxoid matrix
present, three variants of neurothekeoma are described: 1) myxoid,
which is the most common variety, 2) cellular and 3) mixed type. The
classic myxoid type, is encapsulated, characterized by myxomatous
changes, less cellularity with well-circumscribed spindle cells in a
myxoid matrix associated with multinucleated giant cells. The cellular
type is not encapsulated, the cells are epithelioid with eosinophilic
cytoplasm and rare mitosis. Cellular neurothekeomas can be locally
invasive with perineural and vascular invasion, but no locoregional
metastasis. The mixed type of neurothekeoma has varied cellularity with
focal myxoid regions. Clinically, neurothekeomas are slow-growing
asymptomatic lesions, but may be accompanied by pain upon pressure.
Though commonly dermal, mucosa and submucosal lesions have also been
described. Neurothekeomas tend to affect females more often than males,
usually in the second and early third decades of life. Age at
presentation can be between 15 months and 84 years. The most common
location is the upper extremity, followed by the head and neck region,
and lower extremity. Neurothekeoma clinically presents as a
superficially located skin-colored, pink, red, or brown
well-circumscribed papule or nodule measuring less than 2 cm.
Management of neurothekeomas consists of complete surgical excision
with microscopic negative margins. Incomplete removal of the lesion
will lead to recurrence in approximately 15% of patients. There are
reports of multiple neurothekeomas presenting concurrently. It is
almost impossible to make the diagnosis of a neurothekeoma
preoperatively, unless the patient has suffered a previous excision of
such dermal tumor. As neurothekeomas are considered benign lesions, the
prognosis is excellent with very low recurrence rate following complete
surgical excision.
References:
1- Al-Buainain H, Pal K, El Shafie H, Mitra DK, Shawarby MA: Myxoid
neurothekeoma: a rare soft tissue tumor of hand in a 5 month old
infant. Indian J Dermatol. 54(1):59-61, 2009
2- Zenner K, Dahl J, Deutsch G, Rudzinski E, Bly R, Perkins JA:
Metastatic cellular neurothekeoma in childhood. Int J Pediatr
Otorhinolaryngol. 119:86-88, 2019
3- Murphrey M, Huy Nguyen A, White KP, Krol A, Bernert R, Yarbrough K:
Pediatric cellular neurothekeoma: Seven cases and systematic review of
the
literature. Pediatr Dermatol. 37(2):320-325, 2020
4- Massimo JA, Gasibe M, Massimo I, Damilano CP, De Matteo E,
Fiorentino J: Neurothekeoma: Report of two cases in children and review
of the literature. Pediatr Dermatol. 37(1):187-189, 2020
5- Pan HY, Tseng SH, Weng CC, Chen Y: Cellular neurothekeoma of the upper lip in an infant. Pediatr Neonatol. 55(1):71-4, 2014
6- Peñarrocha M, Bonet J, Minguez JM, Vera F: Nerve sheath
myxoma (neurothekeoma) in the tongue of a newborn. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 90(1):74-7, 2000
Renal Medullary Carcinoma
Renal medullary carcinoma (RMC) is a rare and highly
aggressive neoplasm that affects African-American adolescents and young
adult occurring almost exclusively in children with sickle cell trait
or sickle cell hemoglobinopathy. Sickle cell trait is a risk factor for
several conditions including chronic kidney disease, RMC, venous
embolism and sudden death. Most patients present between the ages of 11
and 39 years, with males affected 2:1 ratio. Most common presenting
symptoms for renal medullary carcinoma are gross hematuria, flank
pain, weight loss and abdominal mass. RMC is characterized for early
and widespread metastases. RMC arises from the renal papilla or
calyceal epithelium triggered by chronic medullary hypoxia. Imaging of
RMC commonly identifies a mass, more often in the right-side kidney,
with an average size of 7 cm associated with satellite lesions and
intratumoral necrosis. The most common imaging appearance of RMC is a
poorly marginated, infiltrating mass abutting the pelvocaliceal system.
This tumor is typically associated with a pseudocapsule with
well-defined margins. Lung metastasis have an atypical imaging
appearance with the most common pattern being pulmonary lymphangitic
carcinomatosis and nodules with indistinct margins being more common
than nodules with distinct margins. Pathologically, RMS is an
infiltrative tumor extending from the renal pelvis. These tumors
comprise sheets of poorly differentiated cells commonly found to have a
reticular growth pattern and adenoid cystic component with an
infiltrate of neutrophils. Visualization of sickle red blood cells is
pathognomonic for RMC. Most RMC are associated with a loss of
SMARCB1/INI1 occurring through a chromosomal translocation or deletion
that results in loss of protein expression identifiable by
immunohistochemistry. The management of RMC is radical nephrectomy with
retroperitoneal lymph node dissection followed in most cases by
systemic chemotherapy. At the time of diagnosis 70% of patients have
local lymph node involvement with one site of metastatic disease and
30% have two sites of metastatic involvement, most commonly lymph node,
lung, liver or the contralateral kidney. Cytotoxic chemotherapy with
platinum-based regimens has demonstrated partial and complete responses
with clinical benefit in several case series. No evidence points
to the benefit of screening patients with sickle cell trait for RMC
because no feasible schedule and modality of screening would have an
increase chance of identifying presentation of disease at an early
stage. No effective measure exists for prevention of this type of renal
malignancy. RMC carries a dismal prognosis with less than 5% surviving
longer than 36 months.
References:
1- Beckermann KE, Sharma D, Chaturvedi S, et al: Renal Medullary
Carcinoma: Establishing Standards in Practice. J Oncol Pract.
13(7):414-421, 2017
2- Sandberg JK, Mullen EA, Cajaiba MM, et al: Imaging of renal
medullary carcinoma in children and young adults: a report from the
Children's Oncology Group. Pediatr Radiol. 47(12):1615-1621, 2017
3- Msaouel P, Hong AL, Mullen EA, et al: Updated Recommendations on the
Diagnosis, Management, and Clinical Trial Eligibility Criteria for
Patients With Renal Medullary Carcinoma. Clin Genitourin Cancer.
17(1):1-6, 2019
4- Elliott A, Bruner E: Renal Medullary Carcinoma. Arch Pathol Lab Med.
143(12):1556-1561, 2019 5- Blas L, Roberti J, Petroni J, Reniero L,
Cicora F: Renal Medullary Carcinoma: a Report of the Current
Literature. Curr Urol Rep. 2019 Jan 17;20(1):4. doi:
10.1007/s11934-019-0865-9.
6- Holland P, Merrimen J, Pringle C, Wood LA: Renal medullary carcinoma
and its association with sickle cell trait: a case report and
literature review. Curr Oncol. 27(1):e53-e56, 2020
Epidermolysis Bullosa
Epidermolysis bullosa (EB) is a spectrum of rare, inherited,
blistering bullous skin disorders primarily affecting the skin and
pharyngoesophageal mucosa. EB affects approximately two to 4 per
100,000 children each year in the USA. EB is caused by a mutation in
the genes that encode any of the structural components of keratinocytes
and dermoepithelial junction. The mutation causes changes in proteins
that are responsible for adhesions defects between cutaneous structures
leading to blister formation. Autoantibodies to collagen VII binding to
the anchoring fibril zone and inducing mucocutaneous blistering have
been found in the acquired form of the disease in adults. EB is
classified into four main groups according to location of skin
separation. The four groups include EB simplex (intraepidermal layer),
junctional (within the lamina lucida of the basement membrane),
dystrophic (below the basement membrane) and a mixed type referred as
Kindler syndrome (mixed skin cleavage pattern). EB can be localized or
systemic. Cutaneous findings include blisters, scars, pigmentation
changes, alopecia, absent or dystrophic nails and deformity of hands
and feet. Extracutaneous symptoms of EB might affect eyes, teeth, oral
mucosa, genitourinary, gastrointestinal, respiratory and
musculoskeletal. The diagnosis of EB can be made from a skin biopsy,
genetic mutational analysis or detecting autoantibodies bound to the
basement membrane zone and in the serum against collagen VII.
Gastrointestinal complications of EB include blistering and stenosis of
the esophagus causing dysphagia and weight loss, gastroesophageal
reflux disease, hiatal hernia, gastritis, protein losing enteropathy,
anal fissure, megacolon, inflammatory bowel disease and constipation.
Constipation is one of the most common clinical features of EB
occurring in 40-75% of cases. Constipation occurs when defecation is
painful due to perianal blisters and fissures leading to fecal
retention. Blister formation in the oral mucosa can cause scarring
resulting in microstomia and ankyloglossia which restrict food
ingestion. Teeth might be structurally defective with poor quality
enamel. Anemia is a frequent and serious complication of severe EB. The
more severe the EB types, the more extensive the nutritional
impairment. Feeding via gastrostomy should be initiated before the
onset of malnutrition in order to improve growth recovery, and before
the age of ten to allow pubertal development which has a positive
psychological impact. Pharynx and esophageal strictures are a common
and very morbid complication of EB resulting in dysphagia and
odynophagia. Pneumatic dilatation using a high-pressure hydrostatic
balloon catheter is the gold standard of treatment of symptomatic
esophageal stenosis. Medical therapy of EB can include neutrophil
targeting therapy, immunosuppressors, immunoglobulin, monoclonal
antibodies, tumor necrosis factor inhibition, steroids and gene
therapy.
References:
1- Zidorio AP, Dutra ES, Leao DO, Costa IM: Nutritional aspects of
children and adolescents with epidermolysis bullosa:literature review.
An Bras Dermatol. 90(2):217-23, 2015
2- Kridin K, Kneiber D, Kowalski EH, Valdebran M, Amber KT:
Epidermolysis bullosa acquisita: A comprehensive review. Autoimmun Rev.
18(8):786-795, 2019
3- Mariath LM, Santin JT, Schuler-Faccini L, Kiszewski AE: Inherited
epidermolysis bullosa: update on the clinical and genetic aspects. An
Bras Dermatol. 95(5):551-569, 2020
4- Titeux M, Bonnet des Claustres M, Izmiryan A, Ragot H, Hovnanian A:
Emerging drugs for the treatment of epidermolysis bullosa. Expert Opin
Emerg Drugs. 25(4):467-489, 2020
5- Mortell AE, Azizkhan RG: Epidermolysis bullosa: management of
esophageal strictures and enteric access by gastrostomy. Dermatol Clin.
28(2):311-8, 2010
6- Denyer JE: Wound management for children with epidermolysis bullosa. Dermatol Clin. 28(2):257-64, 2010
 References:
References: