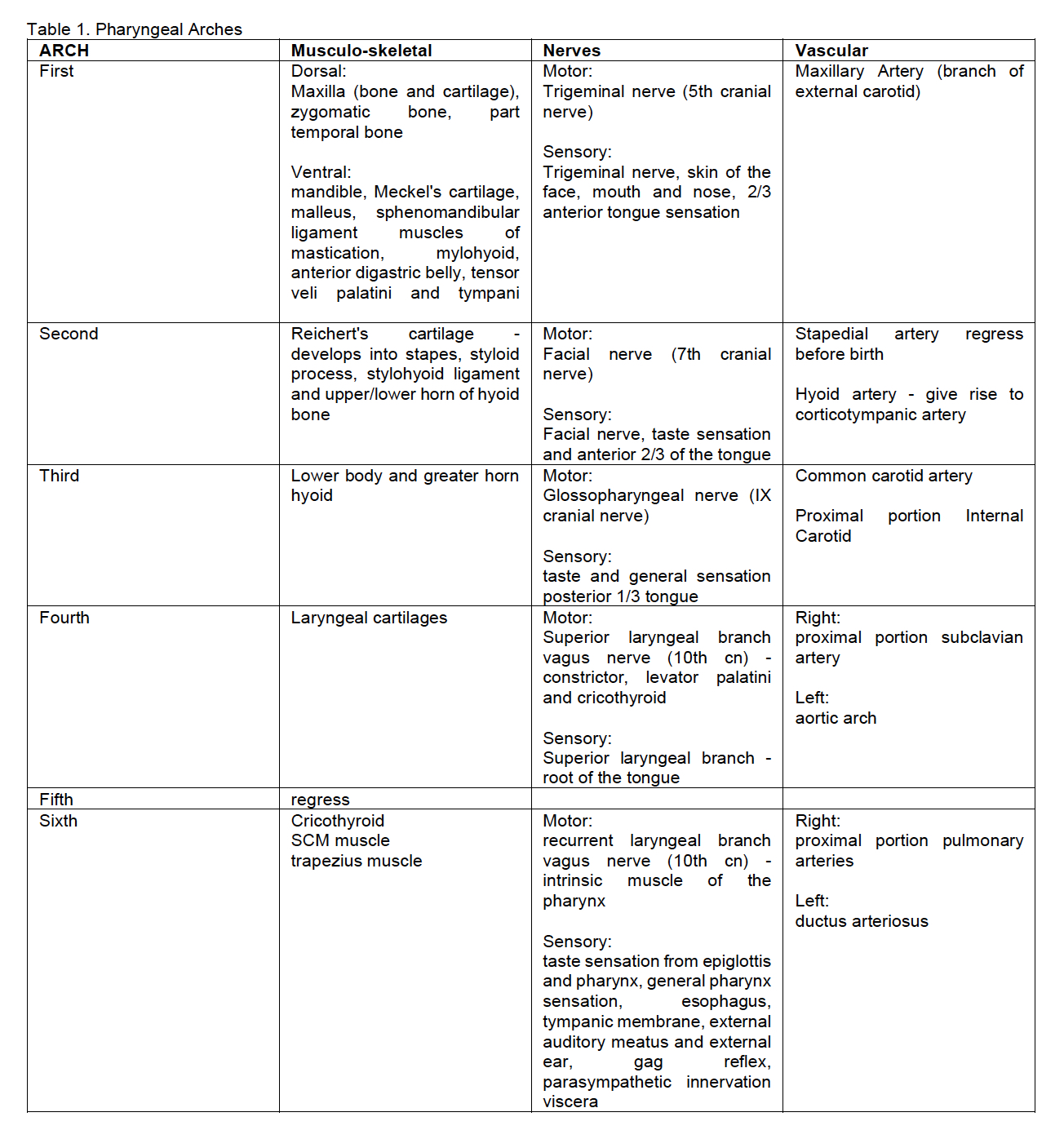
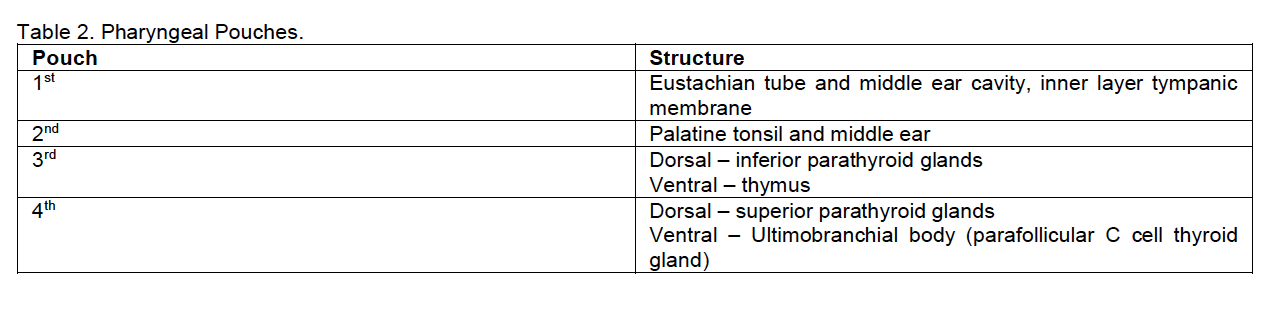
Pediatric germ cell tumors are rare, with 80% benign and 20%
malignant. Ovarian and testicular site accounts for 40% of all cases.
Testicular germ cell tumors are the most common tumors which usually
occur in males between 20-40 years old. The incidence has risen
markedly over the years. Testicular germ cell tumors have two main
histology: seminoma and non-seminomatous. Non-seminomatous GCT includes
embryonal carcinoma, choriocarcinoma, teratoma and yolk sac tumor.
Teratoma, both mature and immature, are the most common histologic
non-seminomatous variety. Germinomas, also term seminoma in males and
dysgerminoma in females, are undifferentiated germ cell tumors typical
of adolescents. Testicular germ cell tumors have two age peaks:
children under three years may experience both mature teratoma and
malignant yolk sac tumor, while adolescents may harbor seminomas or
other mixed tumors often with delayed diagnosis and more advanced
disease. The occurrence of germ cell tumors increases in children with
undescended testes, and the risk is higher with Intraabdominal testes.
The most common histological subtype in this situation is seminoma,
which occur in adolescent and young adults. Patients with Trisomy 21
are 50 times more likely to have testicular cancer. Testicular seminoma
originates in the germinal epithelium of the seminiferous tubules. The
disease is thought to result from the proliferation of immature
spermatogonia. There are three main pathologic categories of testicular
seminoma: classical, spermatocytic and seminoma with
syncytiocytotrophoblastic cells. This last type is associated with
elevated serum beta-HCG levels. A seminoma with a high mitotic index
(>3 mitotic figures/HPF) is designated an anaplastic seminoma, a
more aggressive tumor. Main clinical findings with testicular seminoma
are a painless scrotal mass which may be associated with infertility,
though the tumor may appear as an emergent situation with pain and
acute inflammatory characteristics and hydrocele. On physical exam
there is a unilateral, firm to hard palpable mass in the scrotum
localized to the testis. In a patient with a testicular mass, serum
markers measurements represent the first diagnostic step to verify a
possible malignant germ cell tumor. These include AFP, beta-HCG and
LDH. AFP elevation indicates non-seminomatous disease, LDH is used to
follow the overall seminomatous tumor burden, and beta-HCG is present
in 5-10% of seminoma patents usually associated with metastatic
disease. In addition, Placental alkaline phosphatase (PALP) produced by
the placenta, is also produced by cancer cells. PALP is not specific
for testicular tumors, it can also be found in pulmonary, digestive
system, breast, and female reproductive organ cancer. PALP is commonly
employed as a routine diagnostic marker for seminoma/germinoma. PALP
can be detected in 98% of seminomas, 85% yolk sac tumor and 97%
embryonal carcinomas, but not in teratomas. OCT4 is an ectomere binding
transcription factor detected in tumor germ cells which has pluripotent
potential. It is mostly detected in seminomas, embryonal carcinomas,
dysgerminomas, and germ cell component of gonadoblastoma. OCT4 is
positive in 100% seminomas and negative in normal testicular tissue.
OCT4 is more specific and sensitive that PALP. Ultrasound of the
scrotum is the preferred initial imaging investigation to evaluate any
suspected testicular mass growth. Diagnostic work-up includes a
thoraco-abdominal CT-scan to evaluate possible metastatic spread,
especially on lungs and on the retroperitoneal lymph nodes. Should the
suspected lesion suggest a neoplasm, a surgical approach on the tumor
is recommended as soon as possible. Surgery is the main therapy for
testicular tumors. This includes an inguinal approach with vascular
control before mobilization of the testis. If malignancy is proven by
frozen section examination of a biopsy of the mass, en-bloc resection
of testis and spermatic structures with high ligation of the cord at
the internal inguinal ring is needed (radical inguinal orchiectomy).
Patients with scrotal skin involvement by the tumor and those operated
or biopsied through a scrotal approach should undergo hemiscrotectomy
to ensure local disease control. Retroperitoneal lymph node dissection
is required when enlarged nodes remain after chemotherapy. Inguinal
nodes exploration is indicated only in patients with scrotal
involvement. Seminomatous and non-seminomatous tumors are notable for
their responsiveness to chemotherapy. Seminoma is one of the most
treatable cancer with a survival rate of 98% in early-stage disease.
Testicular seminoma is also sensitive to radiation therapy. Radiation
therapy planning is based on the results of a contrast-enhanced CT of
the chest, abdomen, and pelvis.
References:
1- Cecchetto G: Gonadal germ cell tumors in children and adolescents. J Indian Assoc Pediatr Surg. 19(4):189-94, 2014
2- Zhang T, Ji L, Liu B, Guan W, Liu Q, Gao Y: Testicular germ
cell tumors: a clinicopathological and immunohistochemical analysis of
145 cases. Int J Clin Exp Pathol. 11(9):4622-4629, 2018
3- Singh AP, Tanger R, Mishra D, Ansari M, Gupta AK, Shukla AK:
Testicular Mixed Germ Cell Tumor in a Newborn Child: A Rare Case.
Journal Indian Association of Pediatric Surgeons. 24(2): 144-146, 2019
4- Sanguesa C, Veiga D, Llavador M, Serrano A: Testicular
tumours in children: an approach to diagnosis and management with
pathologic correlation. Insight into imaging, 11(1), 74, 2020
5- Caballero Mora FJ, Munoz Calvo MT, Garci-a Ros M, et al:
[Testicular and paratesticular tumors during childhood and
adolescence]. An Pediatr (Barc). 78(1):6-13, 2013
6- Bahrami A, Ro JY, Ayala AG: An overview of testicular germ cell tumors. Arch Pathol Lab Med. 131(8):1267-80, 2007
Choriocarcinoma
Pediatric germ cell tumors most commonly develop within the
gonad, though extragonadal primary tumors can involve the anterior
mediastinum, retroperitoneum, or the central nervous system.
Choriocarcinoma is a very rare and aggressive germ cell neoplasm of
trophoblastic origin. Choriocarcinoma is a disease seen predominantly
in women, but men can also be affected as part of a mixed germ cell
tumor. Two types of choriocarcinoma have been described: gestational
and non-gestational. Gestational choriocarcinoma is the most common
form, affect only females and arises following a hydatidiform mole,
normal pregnancy or most commonly, spontaneous abortion.
Non-gestational choriocarcinoma is rare, arises from pluripotent germ
cell, can affect young males or females, in the gonads, or midline
structures with pluripotent germ cells. The pathogenesis of
choriocarcinoma start with cytotrophoblastic cells functioning as stem
cells and undergoing malignant transformation. Choriocarcinoma
Histologic demonstrate syncytiotrophoblasts, these are large
eosinophilic multinucleated cells with known large hyperchromatic
nuclei, intermixed with cytotrophoblasts, polygonal cells with distinct
borders and single irregular nuclei. Choriocarcinoma show absence of
chorionic villi and presence of abnormal intermediate trophoblast and
cytotrophoblast, rimmed with syncytiotrophoblasts with areas of
necrosis and hemorrhage. Choriocarcinoma is a extremely vascular
neoplasm characterized by necrosis and absence of chorionic villi. In
mixed germ cell tumors, choriocarcinoma will show mixtures of
syncytiotrophoblasts and cytotrophoblasts with varying component of
other germ cell tumors. Apart from the uterus, it can be found in
tubes, ovaries, lung, liver, spleen, kidneys, bowel, and brain.
Spontaneous abortions and molar pregnancy increase the risk of
developing choriocarcinoma. Due to elevation of HCG, patients can
present with abnormal uterine bleeding, gynecomastia in men and
hyperthyroidism. Males can show symptoms of metastatic disease with
hemoptysis, involved liver, gastrointestinal tract, and brain.
Choriocarcinoma secrete human chorionic gonadotropin (HCG). HCG is an
excellent biomarker of disease progression, response, and subsequent
post-treatment surveillance. After diagnosis of choriocarcinoma
evaluation for malignancy should be undertaken. The lung is the most
common site of metastatic disease. Brain, chest, abdomen, and pelvis
should be evaluated by CT or MRI imaging. Management of choriocarcinoma
entails multimodal therapy with drugs, radiation, and surgery. Low risk
choriocarcinoma can be managed with single agent chemotherapy.
High-risk disease is managed with multi-agent chemotherapy, adjuvant
radiation and surgery. Removal of the uterus or metastatic foci is
performed in conjunction with chemotherapy. The prognosis of
non-gestational choriocarcinoma is worse than his gestational
counterpart due to low sensitivity to chemotherapy. Low risk
choriocarcinoma has a 100% survival in women managed with chemotherapy,
while high-risk tumors have a 90% survival with multiagent therapy with
or without radiation and surgery. Primary infantile or neonatal
choriocarcinoma is extremely rare with most cases involving metastatic
disease to the fetus from an intraplacental choriocarcinoma. Is
associated with a poor prognosis and high mortality for the baby. Most
of these mother with choriocarcinoma were asymptomatic during
pregnancy. Most babies show anemia, developmental delay, hepatomegaly,
hemoptysis, or respiratory failure. Ultrasound shows a tumor with rich
vascularization. Beta-HCG is extremely high. Management is multiagent
chemotherapy. Infantile choriocarcinoma represent a metastatic focus
from primary maternal or placental gestational trophoblastic
tumor.
References:
1- Ngan HYS, Seckl MJ, Berkowitz RS, et al: Update on the
diagnosis and management of gestational trophoblastic disease.
Int J Gynaecol Obstet. 143 Suppl 2:79-85., 2018
2- Keenan C, Ramirez N, Elijovich L, et al: A rare manifestation
of choriocarcinoma syndrome in a child with primary intracranial germ
cell tumor and extracranial metastases: A case report and review of the
literature. Pediatr Blood Cancer. 68(6):e29000, 2021
3- Rzanny-Owczarzak M, Sawicka-Metkowska J, Jonczyk-Potoczna K,
et al: Simultaneous Occurrence of Choriocarcinoma in an Infant and
Mother. Int J Environ Res Public Health. 18(4):1934, 2021
4- Shah R, Weil BR, Weldon CB, Amatruda JF, Frazier AL: Neonatal
Malignant Disorders: Germ Cell Tumors. Clin Perinatol. 48(1):147-165,
2021
5- Ngan HYS, Seckl MJ, Berkowitz RS, et al: Diagnosis and
management of gestational trophoblastic disease: 2021 update. Int
J Gynaecol Obstet.155 Suppl 1(Suppl 1):86-93, 2021
Microaggression in Surgery
Originally described by Chester Pierce in 1970,
microaggression referred to a minor damaging humiliation and indignity
toward African-Americans. Subtle comments, slights and insults directed
toward minorities, as well as to women and other stigmatized groups
that engender hostility. Microaggresion is a brief and commonplace
verbal, behavioral or unintentional environmental discrimination
against a personal characteristic such as gender, sexual orientation,
race, or ethnicity. Microaggressions are associated with a negative
emotional response which may be associated with worsening mental
health. They have a negative effect and unpleasant psychological impact
on recipients. The term microaggression expanded in 2007 to include all
minorities groups as well as marginated communities. Microaggressions
can be a conscious or unconscious microprejudice. Four subtypes of
microaggression have been described: microassault, microinsult,
microinvalidations, and environmental microaggressions. Microassault
are "old fashioned" discriminatory statements, often intentional,
characterized by verbal or nonverbal attacks clearly intended to offend
the recipient. Microassaults are toward individuals rather than a group
with racism motivation. Microinsults are subtle snubs or humiliations
that convey a demeaning message to the recipient in a way that may be
unintentional to the perpetrator. It happens when women or minority
members of society are confused for a nurse, janitor, or interpreter,
because they are not seen as a traditional physician. Ignoring medical
students in the operating room or doing rounds, making comments that
suggest people obtained their current position because of affirmative
action rather than knowledge, skills, or abilities. Microinvalidations
are performed to exclude, negate, dismiss the personal thoughts,
feelings, or experiment reality of a person. They deny concern about
fairness by insisting that the workplace in a meritocracy or
invalidating a woman or minority student experience of inequality by
calling them oversensitive. Environmental microaggressions occur when
microassault, microinsult and microinvalidation are reflected in the
cultural processes and climate of the workplace. An example is a
hallway decorated with pictures of white male surgeons, when medical
schools and departments of surgery unintentionally exclude and minimize
the identity of minorities and women by excluding accomplishments and
portrait of members of some racial, ethnic, and cultural background.
Microaggressions adversely affect the psychological and physical health
of the recipient. Microaggressions produce depression, anxiety,
traumatic response, increase use of alcohol and hypertension in the
recipient. Racism and sexism that manifest as microaggressions in the
workplace present dilemmas for individuals from minorities groups.
Microaggressions can be responded by raising a voice of concern and
resistance, relying on social networks for support, or developing
self-protective mechanisms such as desensitization. A structured
response from the recipient can start with a conversation with the
perpetrator to observe, think, feel and desire how the comment was
interpreted. Next the victim can use action by asking clarifying
questions, tell what you observed, discuss the impact of the comment,
and express your own thought and feeling about the situation. A third
strategy of response is to focus on what was observed and the
recipientsÕ resulting thoughts or feelings to decrease the
potential for defensiveness and encourage dialogue. Subtle racial
comments, actions, and assumptions are witnesses, are experiences by
minority medical students contributing significantly to feelings of
burnout, invalidation, and insecurity in them. Bias is the prejudice in
favor of or against one thing, person, or group. When bias is
unconscious, uncontrollable, or as result of an irrational process, the
bias is implicit. Implicit bias in medical education can result in
inaccurate evaluations that affect promotion and disproportionate
hiring and representation. Microaggression and implicit bias can extend
bidirectionally from patient to physician as well as from physician to
patient. Environmental invalidations are the most common
microaggression reported, suggesting women in surgery face ongoing
microaggressions at the systemic level creating many barriers to
advancement. Implicit bias can manifest in microaggressive actions such
as overlooking women for positions of power or offering challenging
cases to male colleagues who are believed to be more capable than their
female counterpart. Women experience more microaggressions than men
during virtual residency interviews, with the most commonly experienced
microaggression type being environmental. As response, women lower the
ranking and stand out negatively toward those programs committing
microaggressions.
References:
1- Torres MB, Salles A, Cochran A: Recognizing and Reacting to
Microaggressions in Medicine and Surgery. JAMA Surg. 154(9):868-872,
2019
2- Chisholm LP, Jackson KR, Davidson HA, Churchwell AL, Fleming
AE, Drolet BC: Evaluation of Racial Microaggressions Experienced During
Medical School Training and the Effect on Medical Student Education and
Burnout: A Validation Study. J Natl Med Assoc. 113(3):310-314, 2021
3- Turner J, Higgins R, Childs E: Microaggression and Implicit Bias. Am Surg. 87(11):1727-1731, 2021
4- Sprow HN, Hansen NF, Loeb HE, et al: Gender-Based
Microaggressions in Surgery: A Scoping Review of the Global Literature.
World J Surg. 45(5):1409-1422, 2021
5- Hackworth JM, Kotagal M, Bignall ONR, Unaka N, Matheny
Antommaria AH: Microaggressions: Privileged Observers' Duty to Act and
What They Can Do. Pediatrics. 148(6):e2021052758, 2021
6- Hoi KK, Kana LA, Sandhu G, et al: Gender Microaggressions
During Virtual Residency Interviews and Impact on Ranking of Programs
During the Residency Match. J Grad Med Educ. 14(4):398-402, 2022
PSU Volume 59 NO 06 DECEMBER 2022
Nephroptosis
Nephroptosis, floating kidneys or renal ptosis, is defined as a
significant descent of the kidney, of more than 5 cm or two vertebral
bodies, when the patient moves from supine to upright. This is
different to an ectopic kidney where it remains in a constant abnormal
position. The downward displacement of the kidney give rise to symptoms
either due to effects on the ureter or the renal vessels. Nephroptosis
is asymptomatic in most patients (80-90%) and occurs more commonly in
thin women. The condition is rare in the pediatric age, while it
manifest during the second to fourth decade with most being women. The
right side is affected in 70% of cases, the left in 10%, and 20% are
bilateral. It is believed that nephroptosis is caused by excessive
mobility due to deficient support from the perinephric structure. This
can cause stretching, torsion or kinking of the hilar vessels and
proximal ureter. The diagnosis of nephroptosis requires a high index of
suspicion and imaging confirmation. Major symptoms are pain,
nausea/vomiting, transient hematuria, and orthostatic hypertension.
Pain in the flank or abdomen is the most common symptom (90%), and
typically occurs when upright, and relieved by recumbency. Nephroptotic
pain can be associated with intermittent ureteric obstruction causing
hydronephrosis, ischemia, narrowing or kinking of the renal artery or
vein causing stasis, traction and stimulation of the visceral nerves,
and symptoms due to secondary pathology. The hematuria originates from
the calyceal and renal pelvis veins due to compression. Hypertension is
caused by activation of the renin-angiotensin-aldosterone system.
Nephroptosis is a diagnosis of exclusion after ruling out renal
calculi, PUJ obstruction and pyelonephritis. All suspected cases should
undergo both supine and upright studies such as US with Doppler,
intravenous urography, contrast CT and radionuclide scans (dynamic or
static). The Whitaker test, though invasive can also be used as a
diagnostic tool. The affected ptotic kidney will return to its normal
position when the patient is supine. Radionuclear scans (DTPA renogram)
can demonstrate vascular flow impairment, abnormal tubular secretion,
irregular distribution of the tracer, reduced glomerular filtration
rate and outlet obstruction. Management should be considered in
symptomatic patients with more than three months of symptoms duration
and evidence of no other pathology associated, with nuclear medicine
and Doppler imaging showing descent of the symptomatic kidney with
obstruction or diminished flow to the symptomatic side. Treatment is
surgical, namely nephropexy. Nephropexy can be performed open,
laparoscopic, or percutaneously. Optimal nephropexy should include
complete nephrolysis and release of the attachments to the peritoneum,
mobilization of the kidney to a more cephalad retroperitoneal position,
relief of associated urinary obstruction, and fixation of the renal
axis without tension. The percutaneous nephropexy relies in the scar
formation after access and placing a drain for several days. The
laparoscopic nephropexy has a significant successful outcome in more
than 90% of patients, excellent cosmetic results, less postoperative
pain, less hospital stay, lower morbidity and faster recovery. Is more
time-consuming and expensive than the open procedure.
References:
1- Srirangam SJ, Pollard AJ, Adeyoju AA, O'Reilly PH: Nephroptosis: seriously misunderstood? BJU Int. 103(3):296-300, 2009
2- Bansal D, Defoor WR Jr, Noh PH: Pediatric robotic assisted laparoscopic nephropexy: case study. Springerplus. ;2:321, 2013
3- Chan VSH, Lam TPW, Lam WWM: Nephroptosis: The wandering kidney. Kidney Res Clin Pract. 37(3):306-307, 2018
4- Grauer R, Gray M, Schenkman N: Modified Whitaker test: a novel diagnostic for nephroptosis. BMJ Case Rep. 13(4):e235108, 2020
5- Philippou P, Michalakis A, Ioannou K, Miliatou M: Laparoscopic
Nephropexy: The Sliding Clip Technique. J Endourol Case Rep.
17;6(3):224-227, 2020
6- Kahle J, Lakhani J: A disappearing abdominal mass in a teenage
female. SAGE Open Med Case Rep. 2020 Jun 3;8:2050313X20927965. doi:
10.1177/2050313X20927965. eCollection 2020
Pleuropulmonary Blastoma
Pleuropulmonary blastoma (PPB) is considered the most common
primary malignancy of the lung in children. 25-50 new cases are seen
per year in the USA. PPB arise initially as a pulmonary cyst. Three
pathologic stages of PPB have been described: type 1, purely cystic
PPB; type 2 cystic/solid PPB; and type 3 purely solid PPB. The median
age at diagnosis and the pathologic type of PPB: type 1 at 8 months;
type 2 at 35 months; type 3 at 44 months. A progression from type 1 to
3 can occur, though not all cases progress to a more malignant
transformation. Regression of the purely cystic form is called type 1r.
PPB type 1 develops at a much younger age than type 2 or 3, with 97%
presenting before the age of 3 years. Type 1 PPB appears as
multiloculated, air-filled cysts with thin septa. The cysts are lined
with benign respiratory epithelium and mesenchyme, with an underlying
component of malignant mesenchymal cells that may have rhabdomyoblastic
differentiation. Type 1 are most often unilateral, unifocal,
peripheral, over 5 cm in size, and occur with a slight male
predominance. Lung cysts can be seen prenatally between gestational age
23 to 35 weeks. Type 1, purely cystic PPB can be mistaken for another
congenital cystic lung lesion. Type 1r (regression type) was originally
recognize in older relatives of PPB, though cysts with these features
can be found in very young children. Type 1r PPB have the same
multilocular cystic appearance as type 1, but without the interspersed
primitive malignant cells. Type 1r have a median age of diagnosis of 47
months compared to 8 months for type 1. Type 1r age range is
larger, and a lung cyst in an older individual with DICER1 or a
relative with PPB patient is most likely this type. Type 2 PPB account
for approximately one-third of cases, with an equal male-to-female
ratio, and present later than type 1 at a median age of 35 months, very
rarely seen prior to 12 months of age. Type 3 PPB has the worse
prognosis, is entirely comprised of tumor cells without intervening
cystic space, present at a more advanced age, with a median
age-of-diagnosis of 44 months, and do not appear to be seen before 12
months of age. Type 2 and 3 PPB are histologically similar, displaying
a mixed sarcomatous pattern, are diagnosed at an older age, and have
metastatic potential to the brain, bone, and rarely liver.
Chest/abdominal CT and brain MRI with bone scan are required. Both type
2 and 3 are aggressive malignancies that require chemotherapy soon
after the first diagnostic surgery. PPB is associated with an unique
set of disorders, and the genetic basis of the PPB familial syndrome is
the heterozygous loss-of-function mutation of DICER1 found in 70-80% of
children who develop PPB. The presence of such a germline mutation
defines DICER1 PPB familial tumor predisposition syndrome. Germline
DICER1 mutations are inherited as an autosomal dominant fashion in 80%
of cases and arising the novo in the rest. In addition to PPB, DICER1
mutation includes cystic nephroma, ovarian Sertoli-Leydig cell tumors,
ciliary body medulloepithelioma, nodular hyperplasia and differentiated
carcinoma of the thyroid gland, pituitary blastoma, pineoblastoma,
nasal chondromesenchymal hamartoma, and ERMS. Due to the rarity of PPB,
screening for the general population is not needed. In children with
DICER1 mutation only 4% of infants would develop a PPB. DICER1 germline
testing should be performed in all pediatric patients with lung cysts
early in life. P53 mutations in the cystic epithelial cells also have
an important role in PPB type progression. Clinical presentation is
usually with nonspecific respiratory complaints such as difficulty in
breathing, dyspnea, chest pain, hemoptysis. Fever, malaise, and
anorexia are associated with type 2 and 3 PPB. On imaging, chest simple
films are the first modality used for evaluation. PPB is most commonly
seen on the right side. It appears as hemi-opaque thorax with
contralateral tracheal and mediastinal deviation. Diagnosis needs CT of
chest and metastatic workup for type 2 and 3, since metastasis is
unknown in type 1. Pleural effusion and pneumothorax con be commonly
seen. Calcification is not a common finding. Management of PPB is
surgical resection. Type 1 and 1r should be managed via complete
resection with widely negative margins. An open approach is advocated
to minimize chance of tumor spillage. Adjuvant chemotherapy is not
typically given for type 1 PPB unless there is intraoperative tumor
spill, incomplete resection, or local invasion of adjacent structures.
In older asymptomatic patients with type 1r removal is not explicitly
indicated and observation might be appropriate. For type 2 and 3 PPB,
both systemic chemotherapy and surgical resection are critical
component of management. Chemotherapy is typically based on sarcoma
regimens. Surgical resection of type 2 and 3 PPB may require from a
wedge resection to lobectomy or pneumonectomy to achieve negative
margins. Involved pleural space should also be resected en-bloc with
the primary tumor and involved pulmonary lobe. Radiation therapy is
ineffective in general. For brain metastasis all three treatment
modalities, surgery, chemotherapy, and radiation therapy is
recommended. The type of PPB is the strongest prognostic factor, with
outcomes better for type 2 over type 3. Correlation between the PPB
type and survival is type 1 -94%; type 2, 71%; type 3, 53%. Distant
metastasis at diagnosis had a statistically significant detrimental
effect on survival. Anaplasia is common in both type 2 and 3 without
significant prognostic effect. Gross total resection for adequate local
control has important favorable prognostic implication. The PPB type
and presence of distant metastasis at diagnosis are the most important
prognostic factors related to treatment outcome.
References:
1- Messinger YH, Stewart DR, Priest JR, et al: Pleuropulmonary
blastoma: a report on 350 central pathology-confirmed pleuropulmonary
blastoma cases by the International Pleuropulmonary Blastoma Registry.
Cancer. 121(2):276-85, 2015
2- Knight S, Knight T, Khan A, Murphy AJ: Current Management of
Pleuropulmonary Blastoma: A Surgical Perspective. Children (Basel).
6(8):86, 2019
3- Madaan PK, Sidhu HS, Girdhar S, Mann KK: Pleuropulmonary blastoma: A
report of three cases and review of literature. Radiol Case Rep.
16(10):2862-2868, 2021
4- Gonzalez IA, Stewart DR, Schultz KAP, Field AP, Hill DA, Dehner LP:
DICER1 tumor predisposition syndrome: an evolving story initiated with
the pleuropulmonary blastoma. Mod Pathol. 2022 Jan;35(1):4-22. doi:
10.1038/s41379-021-00905-8.
5- Kunisaki SM, Lal DR, Saito JM, et al: Pleuropulmonary Blastoma in
Pediatric Lung Lesions. Pediatrics. 2021 Apr;147(4):e2020028357. doi:
10.1542/peds.2020-028357. Epub 2021 Mar 24.
6- Bownes LV, Hutchins SC, Cardenas AM, Kelly DR, Beierle EA:
Pleuropulmonary blastoma in an adolescent. J Pediatr Surg Case Rep.
59:101482, 2020
7- Sparber-Sauer M, Tagarelli A, Seitz G, et al: Children with
progressive and relapsed pleuropulmonary blastoma: A European
collaborative analysis. Pediatr Blood Cancer. 68(12):e29268, 2021
Sternal Cleft
Sternal cleft is a very rare congenital midline malformation
resulting from failure of fusion of the sternum. It represents 0.15% of
all patients presenting with a chest wall malformation in life. Sternal
cleft arise from failure of development or ventral fusion of the
sternal bars between the 6th and 9th weeks of gestation. The resultant
anatomical defect produces and concave defect in the sternum covered by
skin, with an orthotopic heart and intact pericardium. The shape of the
defect can vary from a narrow V' to a wider U'-shaped
cleft. Sternal cleft is classified within three major groups: cleft
sternum without associated anomalies, thoracic or true ectopia cordis
with varying degree of cleft sternum with the heart outside the chest
wall, and thoracoabdominal ectopia cordis, also referred as Cantrell's
pentalogy. Sternal cleft without associated anomalies is further
classified as either partial (superior or inferior) or complete. With
partial sternal cleft there is skin coverage of the midline defect with
an intact pericardium and a normal diaphragm. The superior cleft is
often associated with malformations like facial hemangioma or abdominal
raphe. The partial inferior form is often associated with ectopia
cordis alone or as part of the pentalogy of Cantrell. Sternal cleft is
easily diagnosed at birth. Sternal cleft causes paradoxical respiratory
movements and increases the risk of harmful events on mediastinal
viscera. Surgical correction is recommended in the neonatal period. The
thoracic wall has a higher compliance at this age. In older patients
the rib cage is stiff and repair is difficult. Sternal cleft are
usually diagnosed during the neonatal period, can also be diagnosed
prenatally, females are affected more commonly than males and the most
represented form is the partial superior type appearing in two-third of
all cases. One-third develop symptoms such as dyspnea, respiratory
distress, and recurrent respiratory tract infections. Two-thirds has an
associated defect, mainly cardiac and vascular. The PHACE syndrome
(posterior fossa brain abnormalities, hemangiomas, cranial vascular
abnormalities, aortic coarctation, cardiac defects, eye abnormalities)
and sternal malformations are seen together. Pectus excavatum can also
be seen associated with sternal cleft. If not treated surgically
patients with sternal cleft can have impaired gas exchange, present
respiratory symptoms such as dyspnea and cough, or develop chest
infection. Contrast enhanced CT of the chest with 3D reconstruction
and/or MRI is utilized as imaging of choices when the defect is
discovered. Echocardiogram is needed to determine cardiac associated
structural defects. Correction by surgery is indicated to protect the
heart and great vessels from direct injury, maintain the growth
potential of the chest wall, avoid use of prosthetic material when
possible, improve respiratory dynamics, and restore a good cosmetic
appearance. Correction during early life permits easy direct closure to
be effected due to flexibility of the chest resulting in a lower risk
of cardiac compression. Primary closure with or without chondrotomies,
periosteal flaps or cartilage resection is the preferred management.
Alternatives procedures include bone graft interposition, prosthetic
closure, and muscle flap interposition. Autologous cartilage from the
resected inferior part of the sternal defect can be used to obliterate
space at the superior part of the sternal defect as it can decrease
tension between the costal ridge during closure. In certain
circumstances a partial or complete thymectomy might be necessary to
reduce the risk of mediastinal compression. Breast development in
female with sternal cleft is normal, but special care must be taken
during repair in neonates not to damage the breast buds. Complications
during surgery include pneumothorax, massive blood loss, cardiac injury
or compression and arrhythmia.
References:
1- Yamanaka K, Higuma T, Watanabe K, Okada Y, Ichida F, Yoshimura N:
Congenital sternal cleft. J Pediatr Surg. 47(11):2143-5, 2012
2- Torre M, Rapuzzi G, Carlucci M, Pio L, Jasonni V: Phenotypic
spectrum and management of sternal cleft: literature review and
presentation of a new series. Eur J Cardiothorac Surg. 41(1):4-9, 2012
3- Harijee A, Vijayaraghavan S, Marathi AR, et al: Complete Sternal Cleft Repair. Indian J Plast Surg. 53(3):419-422, 2020
4- Temur B, Mete S, Beken S, Onalan MA, Erek E: Complete sternal cleft
treatment in a low birth weight patient. Turk Gogus Kalp Damar
Cerrahisi Derg. 28(4):684-687, 2020
5- Tanriverdi HI, Doganeroglu F, Genc A, Yilmaz O: Primary closure of
superior partial sternal cleft in a 2-month-old girl: case report. Ann
Pediatr Surg. 2021;17(1):43. doi: 10.1186/s43159-021-00113-8.
6- Mongkornwong A, Kongpanichakul L, Tawaranurak N, Chansanti O,
Chitithavorn V, Chuangsuwanich A: Autologous tissue reconstruction
sternal cleft: a rare congenital malformation. J Surg Case Rep.
4;2022(3):rjab550. doi: 10.1093/jscr/rjab550, 2022